��Ŀ����
 ��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ��
��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ����1��CaC2��ˮ��Ӧ������Ȳ�ķ�Ӧ����ʽΪ
��2���Ƚϵڶ�����Ԫ��C��N��O����Ԫ�صĵ�һ�����ܴӴ�С˳��Ϊ
��3��CaC��C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ
��4��CaC2����ľ����ṹ��NaCl��������ƣ���ͼ������CaC2�����к��е���������C22-�Ĵ��ڣ�ʹ������һ������������CaC2������1��Ca2+��Χ���������C22-��ĿΪ
��5������Ȳͨ��[Cu��NH3��2]Cl��Һ����Cu2C2����ɫ������Cu+��̬��������Ų�ʽΪ
��6����Ȳ�������ᷴӦ�ɵñ�ϩ�棨H2C=CH-C��N������ϩ�������̼ԭ�ӹ���ӻ�������
���㣺�����ļ���,Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ��,���ȵ���ԭ������Ӧ��,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺��ѧ���뾧��ṹ
��������1�����ݷ�Ӧ���������д����ѧ����ʽ��
��2�����ݵ�һ�����ܵݱ�����жϣ���ϼ۵����Ų�ʽ�жϣ�
��3���ȵ�����Ľṹ���ƣ���O22+�ĵ���ʽ��C22-�ĵ���ʽ���ƣ�����2���м���
��4��������һ��ƽ��ij��������ȣ�����1��Ca2+��Χ���������C22-��4����������6����
��5��CuΪ29��Ԫ�أ�Cu+�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p63d10��
��6����ϩ�棨H2C=CH-C��N���е�C�ֱ��γ�2���ļ���3���ļ�����̼ԭ�ӵ��ӻ��������Ϊsp��sp2�ӻ�����sp�ӻ���Cԭ��ֱ��������ԭ����C��N����ͬ��һ��ֱ������3��ԭ�ӣ�
��2�����ݵ�һ�����ܵݱ�����жϣ���ϼ۵����Ų�ʽ�жϣ�
��3���ȵ�����Ľṹ���ƣ���O22+�ĵ���ʽ��C22-�ĵ���ʽ���ƣ�����2���м���
��4��������һ��ƽ��ij��������ȣ�����1��Ca2+��Χ���������C22-��4����������6����
��5��CuΪ29��Ԫ�أ�Cu+�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p63d10��
��6����ϩ�棨H2C=CH-C��N���е�C�ֱ��γ�2���ļ���3���ļ�����̼ԭ�ӵ��ӻ��������Ϊsp��sp2�ӻ�����sp�ӻ���Cԭ��ֱ��������ԭ����C��N����ͬ��һ��ֱ������3��ԭ�ӣ�
���
�⣺��1����Ӧ��ΪCaC2��H2O��������������Ȳ���ɸ��ݷ�Ӧǰ������д����ѧ����ʽ��CaC2+2H2O�TCH��CH��+Ca��OH��2��
�ʴ�Ϊ��CaC2+2H2O�TCH��CH��+Ca��OH��2��
��2���ڶ����ڵ�һ�����ܴ����ң����������ƣ��ڢ�A��͵ڢ�A�壬��һ�����ֱܷ��Ը��ڵڢ�A��͵ڢ�A�壻CԪ�ؼ۵����Ų�ʽΪ��2s22p2��NԪ�ؼ۵����Ų�ʽΪ��2s22p3��OԪ�ؼ۵����Ų�ʽΪ��2s22p4�����ݺ��ع���������֪��ԭ�ӹ������ȫ�ա��������ȫ����ʱ����Ϊ�ȶ���NԪ�ش��ڰ���������ȶ���
�ʴ�Ϊ��N��O��C��C��N��Oԭ�Ӱ뾶���μ�С��ԭ�Ӻ˶������ӵ�������������ǿ��Ԫ�صĵ�һ��������������Oԭ�����������Ų�Ϊ2s22p4����Nԭ�����������Ų�Ϊ2s22p3��p�����Ų����ڰ����״̬�����ݺ��ع���������֪�������״̬���ȶ�������NԪ�صĵ�һ�����ܱ�O��
��3�����ݵȵ�����ԭ����֪��O22+�ĵ���ʽ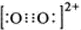 ����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м����ʴ�Ϊ��
����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м����ʴ�Ϊ��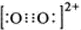 ��2NA��
��2NA��
��4�����ݾ���ʾ��ͼ���Կ�����������һ��ƽ��ij��������ȣ�����ͼ�����Ŀ�֪1��Ca2+��Χ���������C22-��4����������6����Ҫ�ر�ע���������Ϣ���ʴ�Ϊ��4��
��5��CuΪ29��Ԫ�أ�Ҫע��3d���д��4s�����ǰ��ͬʱ���о�������3d�ṹ��Cu+�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p63d10���ʴ�Ϊ��1s22s22p63s23p63d10��
��6��ͨ����ϩ��Ľṹ����֪��̼ԭ�ӵ��ӻ��������Ϊsp��sp2�ӻ�����sp�ӻ���Cԭ��ֱ��������ԭ����C��N����ͬ��һ��ֱ������3��ԭ�ӣ��ʴ�Ϊ��sp�ӻ���sp2�ӻ���3��
�ʴ�Ϊ��CaC2+2H2O�TCH��CH��+Ca��OH��2��
��2���ڶ����ڵ�һ�����ܴ����ң����������ƣ��ڢ�A��͵ڢ�A�壬��һ�����ֱܷ��Ը��ڵڢ�A��͵ڢ�A�壻CԪ�ؼ۵����Ų�ʽΪ��2s22p2��NԪ�ؼ۵����Ų�ʽΪ��2s22p3��OԪ�ؼ۵����Ų�ʽΪ��2s22p4�����ݺ��ع���������֪��ԭ�ӹ������ȫ�ա��������ȫ����ʱ����Ϊ�ȶ���NԪ�ش��ڰ���������ȶ���
�ʴ�Ϊ��N��O��C��C��N��Oԭ�Ӱ뾶���μ�С��ԭ�Ӻ˶������ӵ�������������ǿ��Ԫ�صĵ�һ��������������Oԭ�����������Ų�Ϊ2s22p4����Nԭ�����������Ų�Ϊ2s22p3��p�����Ų����ڰ����״̬�����ݺ��ع���������֪�������״̬���ȶ�������NԪ�صĵ�һ�����ܱ�O��
��3�����ݵȵ�����ԭ����֪��O22+�ĵ���ʽ
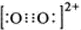 ����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м����ʴ�Ϊ��
����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м����ʴ�Ϊ��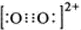 ��2NA��
��2NA����4�����ݾ���ʾ��ͼ���Կ�����������һ��ƽ��ij��������ȣ�����ͼ�����Ŀ�֪1��Ca2+��Χ���������C22-��4����������6����Ҫ�ر�ע���������Ϣ���ʴ�Ϊ��4��
��5��CuΪ29��Ԫ�أ�Ҫע��3d���д��4s�����ǰ��ͬʱ���о�������3d�ṹ��Cu+�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p63d10���ʴ�Ϊ��1s22s22p63s23p63d10��
��6��ͨ����ϩ��Ľṹ����֪��̼ԭ�ӵ��ӻ��������Ϊsp��sp2�ӻ�����sp�ӻ���Cԭ��ֱ��������ԭ����C��N����ͬ��һ��ֱ������3��ԭ�ӣ��ʴ�Ϊ��sp�ӻ���sp2�ӻ���3��
�����������ԡ��л��ϳɹ�ҵ��һ��ԭ����Ȳ��Ϊ�����زģ��������ʽṹ�����ʡ�ѡ�γ�ģ�����������ں���һ�壬�ֱ���ѧ���Եȵ����塢Cu+��̬��������Ų���ԭ�ӹ���ӻ����͡����ӿռ�ṹ��NaCl�������Ƶ�CaC2����ľ����ṹ��1mol O22+�Цм�����Ŀ��֪ʶ�����պ�Ӧ��������

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ
��Na2S��Һ��c��Na+����c��S2-���ı�ֵ�ǣ�������
| A��2 | B����2 |
| C������2 | D��1/2 |
�����Ȼ�ѧ����ʽ��д��ȷ���ǣ���H�ľ���ֵ����ȷ����������
| A��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��g����H=-1367.0kJ/mol��ȼ���ȣ� |
| B��S��s��+O2��g��=SO2��g����H=-269.8kJ/mol����Ӧ�ȣ� |
| C��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=+57.3kJ/mol���к��ȣ� |
| D��Fe+S=FeS��H=-95.6kJ/mol����Ӧ�ȣ� |
��֪I-��Fe2+��SO2��Cl-���л�ԭ�ԣ����ǻ�ԭ�Ե�ǿ��˳���ǣ�SO2��I-��Fe2+��Cl-�������з�Ӧ���ܷ������ǣ�������
| A��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+ |
| B��I2+SO2+2H2O=H2SO4+2HI |
| C��SO2+2Cl-+2H2O=S��+Cl2��+4OH- |
| D��2Fe3++2I-=2Fe2++I2 |
����˵����ȷ���ǣ�������
| A��һ�������£�ʹ�ô����ܼӿ췴Ӧ���ʲ���߷�Ӧ���ƽ��ת���� |
| B��ˮ�ⷴӦNH4++H2O?NH3?H2O+H+�ﵽƽ��������¶�ƽ�������ƶ� |
| C��Ǧ���طŵ�ʱ�ĸ����ͳ��ʱ��������������ԭ��Ӧ |
| D�����ڷ�Ӧ2H2O2=2H2O+O2��������MnO2�������¶ȶ��ܼӿ�O2���������� |

