��Ŀ����
17��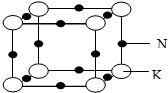 2015��8��12�Žӽ���ҹʱ�֣�����������һ����װ����ͷ������ը��������ը���Ǽ�װ���ڵ���ȼ�ױ���Ʒ����ը������죬��������Ģ���ƣ��������յ���Ϣ������װ������Σ�ջ�ѧƷ�����мء��ơ������ơ�����ء��ռ��軯�ơ�������ϩ���ȵ���ȣ��˵�����Σ�ջ�ѧƷ�����л����������������ᡢ����李��軯�ơ�4��6-��������-���ٶ������ӵȣ�
2015��8��12�Žӽ���ҹʱ�֣�����������һ����װ����ͷ������ը��������ը���Ǽ�װ���ڵ���ȼ�ױ���Ʒ����ը������죬��������Ģ���ƣ��������յ���Ϣ������װ������Σ�ջ�ѧƷ�����мء��ơ������ơ�����ء��ռ��軯�ơ�������ϩ���ȵ���ȣ��˵�����Σ�ջ�ѧƷ�����л����������������ᡢ����李��軯�ơ�4��6-��������-���ٶ������ӵȣ��ش��������⣺
��1�������NH4NO3��NaCN�������ʵ�Ԫ���е�һ������������N����Ԫ�ط��ţ�����ԭ��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ�����Ԫ�صģ�
��2����������ͼ����У���ˮ���ܽ�Ƚϴ���Ǽ��ᣨ�����ƣ���ԭ���Ǽ�����ˮ�γ�������ռ������ľ�������Ϊ���Ӿ��壻�Na2S����S2-�Ļ�̬�����Ų�ʽ��1s2s22p63s23p6��
��3��������У�NO3-�����幹��Ϊƽ�������Σ�����ԭ�ӵ��ӻ��������Ϊsp2��
��4��1mol������NaCN��CN-�����Ħм���Ϊ2NA����CN-��Ϊ�ȵ�����ķ�����CO��N2��
��CN��2�ֳ�Ϊ��±�أ�ʵ���ҿ������軯�ơ��������̺�Ũ�����ڼ����������Ƶã�д�ɸ��Ʊ��Ļ�ѧ����ʽ2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$��CN��2+Na2SO4+MnSO4+2H2O��
��5���ƼغϽ����ڽ������壬��ij�ֺϽ�ľ����ṹ��ͼ��ʾ���Ͻ�Ļ�ѧʽΪKNa3��������K ԭ�ӵ���λ��Ϊ6����֪����ԭ�Ӱ뾶r��Na��=186pm��r��K��=227pm�����㾧��Ŀռ�������$\frac{\frac{4}{3}�У�186{\;}^{3}��3+227{\;}^{3}��}{��186��2+227��2��^{3}}$��100%���г�����ʽ������Ҫ������������
���� ��1��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
��2����ˮ�����γ�������������ʵ��ܽ�ȣ��ռ��������Ӻ����������ɣ��������Ӿ��壻S2-���Ӻ��������Ϊ18�������������ԭ������д���̬�����Ų�ʽ��
��3��NO3-�����е�ԭ�ӵŵ��Ӷ���=$\frac{5+1-2��3}{2}$=0���۲���Ӷ���=3+0=3��
��4��ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ���ӣ�CN-��N2��Ϊ�ȵ����壬CN-�к���C��N�����������к���1���Ҽ���2���м�����������ȡ�����ķ�Ӧ��֪���軯�ơ��������̺�Ũ�����ڼ����������Ƶã�CN��2�������������̡���������ˮ��
��5�����ݾ�̯�����㾧����Na��Kԭ����Ŀ��ȷ���Ͻ�Ļ�ѧʽ�����ݾ���ͼ��֪��ÿ��K ԭ����Χ��6����ԭ�ӣ����ݾ����Ľṹ��֪�������ı߳�Ϊ��ԭ�Ӻͼ�ԭ�ӵ�ֱ��֮�ͣ�����Ŀռ�������Ϊ$\frac{������Na��Kԭ�������}{�������}$��100%��
��� �⣺��1��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ�����Ԫ�صģ���NH4NO3��NaCN�������ʵ�Ԫ���е�һ������������N��
�ʴ�Ϊ��N��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ�����Ԫ�صģ�
��2��������ˮ�γ�����������������ܣ��ʶ�������ͼ������ܽ�Ƚϴ���Ǽ��ᣬ�ռ��������Ӻ����������ɣ������ռ������Ӿ��壬S2-���Ӻ�����18�����ӣ����̬�����Ų�ʽΪ1s2s22p63s23p6��
�ʴ�Ϊ�����������ˮ�γ���������Ӿ��壻1s2s22p63s23p6��
��3��NO3-�����е�ԭ�ӵŵ��Ӷ���=$\frac{5+1-2��3}{2}$=0���۲���Ӷ���=3+0=3������NO3-���幹��Ϊƽ�������Σ�����ԭ�ӵ�ԭ�ӵ��ӻ��������sp2��
�ʴ�Ϊ��ƽ�������Σ�sp2��
��4��ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ���ӣ�CN-��N2��Ϊ�ȵ����壬���߽ṹ���ƣ�CN-�к���C��N�����������к���1���Ҽ���2���м���������1mol������NaCN��CN-�����Ħм���Ϊ2NA��CN-�к�������ԭ�ӡ�10���۵��ӣ���CN-��Ϊ�ȵ�����ķ�����CO��N2��
������ȡ�����ķ�Ӧ��֪���軯�ơ��������̺�Ũ�����ڼ����������Ƶã�CN��2����Ӧ��ѧ����ʽΪ��2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$��CN��2+Na2SO4+MnSO4+2H2O��
�ʴ�Ϊ��2NA��CO��N2��2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$��CN��2+Na2SO4+MnSO4+2H2O��
��5�������У���ԭ����Ϊ12��$\frac{1}{4}$=3����ԭ����Ϊ8��$\frac{1}{8}$=1�����ԺϽ�Ļ�ѧʽΪKNa3��
���ݾ���ͼ��֪��ÿ��K ԭ����Χ��6����ԭ�ӣ����Ծ�����K ԭ�ӵ���λ��Ϊ6��
��������ԭ�Ӻͼ�ԭ�����֮��Ϊ$\frac{4}{3}$��[��186pm��3��3+��227pm��3]�������ı߳�Ϊ��ԭ�Ӻͼ�ԭ�ӵ�ֱ��֮��Ϊ2����186pm+227pm�������Ծ��������Ϊ��2��186pm+2��227pm��3������Ŀռ�������Ϊ{$\frac{4}{3}$��[��186pm��3��3+��227pm��3]�£�2��186pm+2��227pm��3}��100%=$\frac{\frac{4}{3}�У�186{\;}^{3}��3+227{\;}^{3}��}{��186��2+227��2��^{3}}$��100%��
�ʴ�Ϊ��KNa3��6��$\frac{\frac{4}{3}�У�186{\;}^{3}��3+227{\;}^{3}��}{��186��2+227��2��^{3}}$��100%��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰�����ܡ���������Ų����ӻ���ʽ��ռ乹�͡��ȵ����塢��������ȣ��Ѷ��еȣ�������ѧ���ķ��������ͼ��������Ŀ��飬ע�����֪ʶ���������գ�

| A�� | ��0.1mol/LNaHCO3 ��Һ���У�c��Na+����c��HCO3-��+c��CO32-��+c��OH-�� | |
| B�� | ��NaHSO4��Һ���У�c��Na+��=c��SO42-��=c��H+�� | |
| C�� | ��������ˮ�м���KOH����Һ�����ԣ�����Һ���У�c��K+��=2c��ClO-��+C��HClO�� | |
| D�� | �������ʵ���Ũ�ȵ�Na2SO3��Һ��NaHSO4��Һ�������Ϻ�Ļ����Һ�У�c��OH-��-c��H2SO3��=c��H+��+c��SO32-�� |
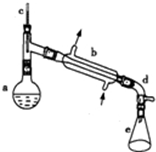 ����ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ����ͼ��ʾ�������õ����й��������£�
����ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ����ͼ��ʾ�������õ����й��������£�| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | �ܽ��� | |
| ���Ҵ� | 100 | 0.9618 | 161 | ����ˮ |
| ����ϩ | 82 | 0.8102 | 83 | ������ˮ |
�ش��������⣺
��1���ɻ�������ȡ����ϩ�ķ���ʽ
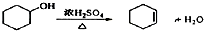 ��
����2��װ��b������ˮ�������½��ϳ������½��ϳ����Ͻ��³�����
��3���������Ƭ�������Ƿ�ֹ���У��������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������B������ȷ�𰸱�ţ���
A���������� B����ȴ�� C�����貹�� D����������
��4����ʵ���������ײ����ĸ����������Ĺ���������Ϊ�Ѽ���
��5���ڷ����ᴿ�У�ʹ�õ�������f�����Ƿ�Һ©���������Ȼ��ƵIJ����ǹ��ˣ�
��6���ϳɹ����м���Ũ����������Ǵ�������ˮ����
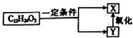 ����ʽΪC12H24O2��������һ�������·�����ͼ��ʾ��ת����������������������Ľṹ�����У�������
����ʽΪC12H24O2��������һ�������·�����ͼ��ʾ��ת����������������������Ľṹ�����У�������| A�� | 4�� | B�� | 6�� | C�� | 8�� | D�� | 10�� |
| A�� | �����ŷŵ�β�� | B�� | ���᳧β�� | C�� | ����װ���� | D�� | ±�����ӷ� |
| A�� | NH3�ڸ÷�Ӧ������ԭ���������� | |
| B�� | Si3N4����Ӳ�ȴ��۵�ߣ�˵��Si3N4���������²��� | |
| C�� | ��״���£�22.4LNH3����ԭ������ĿΪ4��6.02��1023 | |
| D�� | �÷�Ӧÿ����1mol Si3N4ת�Ƶ�������ĿΪ12��6.02��1023 |
| A�� | �ڷ�����������һ�������ɸ�CH2ԭ���ŵ�����һ������Ϊͬϵ�� | |
| B�� | ����ͬ���칹������л������ﻥ��Ϊͬ���칹�� | |
| C�� | ͬ����������ָͬ��Ԫ���γɲ�ͬ�ĵ��� | |
| D�� | ������ͬ������������ͬ����������ͬһ��Ԫ�ص�ԭ�ӻ���Ϊͬλ�� |
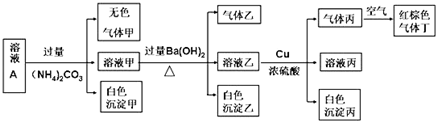
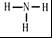 ��
��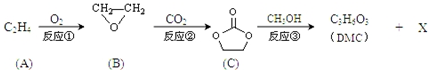
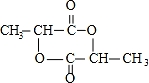 ��
��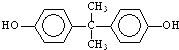 ����һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ�����ɷ������̼������д����Ӧ�Ļ�ѧ����ʽ��2n
����һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ�����ɷ������̼������д����Ӧ�Ļ�ѧ����ʽ��2n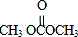 +n
+n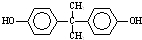 ��
�� +4nCH3OH��
+4nCH3OH��