��Ŀ����
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
��1�������к���һ����̼������X��Fe3C����X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ����������� ��X�����Ũ���ᷴӦ����Һ�к��е��εĻ�ѧʽΪ ��
��2��ij��Һ����Mg2+��Fe2+��A13+��Cu2+���������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ��������� ��
A��Mg2+B��Fe2+C��A13+D��Cu2+
��3������������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�

�ش��������⣺
�ٲ���I�������� ������II�������� ������II��
����Ϊ ��
��Na2CO3��Һ���Գ����ۣ�ԭ���ǣ������ӷ���ʽ��ʾ�� ��
�����������FeCO3���������ӷ���ʽ ��
��4����Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ
��5Fe2++MnO-4+8H+�T5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬���� ��
�ڸ�ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������ ��
A��ϡ����B��ϡ����C��ϡ����D��Ũ����
��ijͬѧ��Ƶ����еζ���ʽ����������� �����гֲ�����ȥ��������ĸ��ţ�

��1�������к���һ����̼������X��Fe3C����X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������
��2��ij��Һ����Mg2+��Fe2+��A13+��Cu2+���������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������
A��Mg2+B��Fe2+C��A13+D��Cu2+
��3������������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�

�ش��������⣺
�ٲ���I��������
����Ϊ
��Na2CO3��Һ���Գ����ۣ�ԭ���ǣ������ӷ���ʽ��ʾ��
�����������FeCO3���������ӷ���ʽ
��4����Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ
��5Fe2++MnO-4+8H+�T5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����
�ڸ�ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������
A��ϡ����B��ϡ����C��ϡ����D��Ũ����
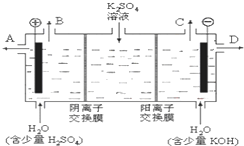
��ijͬѧ��Ƶ����еζ���ʽ�����������

���㣺��������Ԫ�صĵ��ʼ��仯������ۺ�Ӧ��,̽�����ʵ���ɻ�������ʵĺ���,�Ʊ�ʵ�鷽�������
ר�⣺ʵ��̽�������ݴ�����,ʵ�������
��������1��Fe3C�������Ŀ����и������գ������д��ԵĹ���Y��YΪFe3O4������������ᷴӦ�����Ȼ��������Ȼ�������Һ��ʣ���HCl��Ũ�������ǿ�����Խ����������������ӣ������Ũ���ᷴӦ����Һ�к������������������
��2�����������NaOH��Al3+ת��Ϊƫ�������Mg2+��Fe2+��Cu2+ת��Ϊ��������������������������ױ����������չ���õ�����þ������þ�����������ù�����ϡ�����У�������Һ�к���Mg2+��Fe3+��Cu2+��
��3�����ɹ������̿�֪������I�ǽ�������Һ����룬Ӧ��ȡ�����ķ�����FeCO3�����ḽ���������ӣ���Ҫ����ϴ�ӣ��������ʣ�
��Na2CO3��Һ���Գ����ۣ�ԭ����̼������Һˮ��ʼ��ԣ�
�۹�����NH4HCO3�������������HCO3-��HCO3-?H++CO32-����Һ�е��������Ӻʹ�����̼������ӽ��FeCO3������
��4��������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����250 mL����ƿ��
���ữKMnO4��Һ���᱾�����ܾ��л�ԭ�ԣ�����л�ԭҪ�������������������Ҳ���ܾ���ǿ�����ԣ�����ֻ��ѡ���
��������Һ��ǿ��������ҺӦ����ʽ�ζ��ܣ�
��2�����������NaOH��Al3+ת��Ϊƫ�������Mg2+��Fe2+��Cu2+ת��Ϊ��������������������������ױ����������չ���õ�����þ������þ�����������ù�����ϡ�����У�������Һ�к���Mg2+��Fe3+��Cu2+��
��3�����ɹ������̿�֪������I�ǽ�������Һ����룬Ӧ��ȡ�����ķ�����FeCO3�����ḽ���������ӣ���Ҫ����ϴ�ӣ��������ʣ�
��Na2CO3��Һ���Գ����ۣ�ԭ����̼������Һˮ��ʼ��ԣ�
�۹�����NH4HCO3�������������HCO3-��HCO3-?H++CO32-����Һ�е��������Ӻʹ�����̼������ӽ��FeCO3������
��4��������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����250 mL����ƿ��
���ữKMnO4��Һ���᱾�����ܾ��л�ԭ�ԣ�����л�ԭҪ�������������������Ҳ���ܾ���ǿ�����ԣ�����ֻ��ѡ���
��������Һ��ǿ��������ҺӦ����ʽ�ζ��ܣ�
���
�⣺��1��Fe3C�������Ŀ����и������գ������д��ԵĹ���Y��YΪFe3O4�����ڹ��������ᷴӦ�����Ȼ��������Ȼ�������Һ��ʣ���HCl����Һ�д������ڵ���������Fe2+��Fe3+��H+���ʴ�Ϊ��Fe2+��Fe3+��H+��
��2�����������NaOH��Al3+ת��Ϊƫ�������Mg2+��Fe2+��Cu2+ת��Ϊ��������������������������ױ����������չ���õ�����þ������þ�����������ù�����ϡ�����У�������Һ�к���Mg2+��Fe3+��Cu2+����ԭ��Һ��ȣ���Һ�д������ٵ���������Al3+��Fe2+��
�ʴ�Ϊ��B��C��
��3�����ɹ������̿�֪������I�ǽ�������Һ����룬������Һ�������ʵIJ���Ϊ���ˣ�Ӧ��ȡ���˵ķ�����FeCO3�����ḽ���������ӣ���Ҫ����ϴ�ӣ��������ʣ������ڸ���֮ǰ��ϴ�ӣ�����ϴ�ӵķ�������©���м���������ˮ��û���������˸ɺ��ظ�����2��3�Σ�
�ʴ�Ϊ�����ˣ�ϴ�ӣ���©���м�����������ˮ����û��������������ˮ��Ȼ���£��ظ����Σ�
��Na2CO3��Һ���Գ����ۣ����۵ijɷ������ڼ������������ɿ��ܽ�����κʹ���ԭ����̼������Һˮ��ʼ��ԣ��ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
�۹�����NH4HCO3�������������HCO3-��HCO3-?H++CO32-����Һ�е��������Ӻʹ�����̼������ӽ��FeCO3�������������ӷ���ʽΪ��Fe2++2HCO-3�TFeCO3��+CO2��+H2O���ʴ�Ϊ��Fe2++2HCO3-�TFeCO3��+CO2��+H2O��
��4���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����250 mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
���ữKMnO4��Һ���᱾�����ܾ��л�ԭ�ԣ�����л�ԭҪ�������������������Ҳ���ܾ���ǿ�����ԣ�����ֻ��ѡ���ᣬ�ʴ�Ϊ�����
��������Һ��ǿ��������ҺӦ����ʽ�ζ��ܣ��ʴ�Ϊ��b��
��2�����������NaOH��Al3+ת��Ϊƫ�������Mg2+��Fe2+��Cu2+ת��Ϊ��������������������������ױ����������չ���õ�����þ������þ�����������ù�����ϡ�����У�������Һ�к���Mg2+��Fe3+��Cu2+����ԭ��Һ��ȣ���Һ�д������ٵ���������Al3+��Fe2+��
�ʴ�Ϊ��B��C��
��3�����ɹ������̿�֪������I�ǽ�������Һ����룬������Һ�������ʵIJ���Ϊ���ˣ�Ӧ��ȡ���˵ķ�����FeCO3�����ḽ���������ӣ���Ҫ����ϴ�ӣ��������ʣ������ڸ���֮ǰ��ϴ�ӣ�����ϴ�ӵķ�������©���м���������ˮ��û���������˸ɺ��ظ�����2��3�Σ�
�ʴ�Ϊ�����ˣ�ϴ�ӣ���©���м�����������ˮ����û��������������ˮ��Ȼ���£��ظ����Σ�
��Na2CO3��Һ���Գ����ۣ����۵ijɷ������ڼ������������ɿ��ܽ�����κʹ���ԭ����̼������Һˮ��ʼ��ԣ��ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
�۹�����NH4HCO3�������������HCO3-��HCO3-?H++CO32-����Һ�е��������Ӻʹ�����̼������ӽ��FeCO3�������������ӷ���ʽΪ��Fe2++2HCO-3�TFeCO3��+CO2��+H2O���ʴ�Ϊ��Fe2++2HCO3-�TFeCO3��+CO2��+H2O��
��4���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����250 mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
���ữKMnO4��Һ���᱾�����ܾ��л�ԭ�ԣ�����л�ԭҪ�������������������Ҳ���ܾ���ǿ�����ԣ�����ֻ��ѡ���ᣬ�ʴ�Ϊ�����
��������Һ��ǿ��������ҺӦ����ʽ�ζ��ܣ��ʴ�Ϊ��b��
���������⿼��Ԫ�ػ��������ʡ���ѧ�������̡����������뷽����ơ�����ˮ�⡢���û�ѧ����ȣ��Ѷ��еȣ��Ƕ���ѧ֪ʶ���ۺ����ã�Ϊ�߿��������ͣ�ע���ϵʽ�������ã�

��ϰ��ϵ�д�
�����Ŀ
�谢���ӵ���������ֵΪ6.02��1023������˵����ȷ���ǣ�������
| A���ڱ�״���£�11.2L N2��NO�Ļ����������ԭ����Ϊ 6.02��1023 |
| B����״���£�32g O2��O3�Ļ����������ԭ����ĿΪ6.02��1023 |
| C��0.5 mol/L��CaCl2��Һ��Cl-����ĿΪ6.02��1023 |
| D��ͨ�������£�7.1g 37Cl2��������ĿΪ4.0��6.02��1023 |
�����йط�ӦCl2+H2O=HCl+HClO�������У���ȷ���ǣ�������
| A��Cl2ֻ�������� |
| B��Cl2ֻ����ԭ�� |
| C��Cl2����������������ԭ�� |
| D����Ԫ�صĻ��ϼ۲������仯 |
 Na2CO3��һ�ֺ���Ҫ�Ļ�ѧ���ʣ�ijѧ������ʵ�������Ʊ�Na2CO3�������������Ʊ�ʵ����̣���50mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�������������NaHCO3�������������ʵ�鲽�裺
Na2CO3��һ�ֺ���Ҫ�Ļ�ѧ���ʣ�ijѧ������ʵ�������Ʊ�Na2CO3�������������Ʊ�ʵ����̣���50mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�������������NaHCO3�������������ʵ�鲽�裺