��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش��������⣺

��1���ܡ��ݡ������Ӱ뾶�ɴ�С��˳���� ��д���ӷ��ţ���
��2���ۡ��ߡ������ۺ������������ǿ������˳���� ��д��ѧʽ����
��3���ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д�������ʽ�� ��
��4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ����X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ��


��1���ܡ��ݡ������Ӱ뾶�ɴ�С��˳����
��2���ۡ��ߡ������ۺ������������ǿ������˳����
��3���ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д�������ʽ��
��4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ����X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ

���㣺λ�ýṹ���ʵ����ϵӦ��,Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
��������Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl����ΪBe��
��1�����Ӳ�Խ�࣬���Ӱ뾶Խ������ͬ�Ų������ӣ�ԭ������������Ӱ뾶С��
��2��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ����ۺ����������Խǿ��
��3���ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�����Na2O2��
��4��M�ǽ����ǽ�����������һ������Σ����Z
N���ĵ��ʿ�֪�����ǽ������������ƶ�N����������Z��������������X+Y+H2O��Al��OH��3+NH4+���Դ������
��1�����Ӳ�Խ�࣬���Ӱ뾶Խ������ͬ�Ų������ӣ�ԭ������������Ӱ뾶С��
��2��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ����ۺ����������Խǿ��
��3���ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�����Na2O2��
��4��M�ǽ����ǽ�����������һ������Σ����Z
| �� |
���
�⣺��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl����ΪBe��
��1�����Ӳ�Խ�࣬���Ӱ뾶Խ������ͬ�Ų������ӣ�ԭ������������Ӱ뾶С����ܡ��ݡ������Ӱ뾶�ɴ�С��˳����O2-��Na+��Al3+��
�ʴ�Ϊ��O2-��Na+��Al3+��
��2��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ����ۺ����������Խǿ���ǽ�����Cl��Si��N��Si������Ϊ��������������ǿ���ᣬ��ۡ��ߡ������ۺ������������ǿ������˳����HClO4��HNO3��H2SiO3���ʴ�Ϊ��HClO4��HNO3��H2SiO3��
��3���ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�����Na2O2�������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��M�ǽ����ǽ�����������һ������Σ����Z
N���ĵ��ʿ�֪�����ǽ������������ƶ�N����������Z��������������X+Y+H2O��Al��OH��3+NH4+����X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3+3H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3+3H2O=Al��OH��3��+3NH4+��
��1�����Ӳ�Խ�࣬���Ӱ뾶Խ������ͬ�Ų������ӣ�ԭ������������Ӱ뾶С����ܡ��ݡ������Ӱ뾶�ɴ�С��˳����O2-��Na+��Al3+��
�ʴ�Ϊ��O2-��Na+��Al3+��
��2��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ����ۺ����������Խǿ���ǽ�����Cl��Si��N��Si������Ϊ��������������ǿ���ᣬ��ۡ��ߡ������ۺ������������ǿ������˳����HClO4��HNO3��H2SiO3���ʴ�Ϊ��HClO4��HNO3��H2SiO3��
��3���ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�����Na2O2�������ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4��M�ǽ����ǽ�����������һ������Σ����Z
| �� |
���������⿼��Ԫ��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ϊ��Ƶ���㣬ע����ճ���Ԫ�ؼ��仯�������ʡ������ʵıȽϡ���ѧ�����γɺʹ��ڡ������仯����Ļ�ѧ���ʡ����������Ʊ���֪ʶ�ƶ����ʣ��ۺ��Խ�ǿ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
��֪����һ±�����ڴ����������¿����ɱ���ͬϵ� �ڴ����������£��ɱ������и������ʺϳ��ұ������Ӧѡ�õ��ǣ�������
�ڴ����������£��ɱ������и������ʺϳ��ұ������Ӧѡ�õ��ǣ�������
 �ڴ����������£��ɱ������и������ʺϳ��ұ������Ӧѡ�õ��ǣ�������
�ڴ����������£��ɱ������и������ʺϳ��ұ������Ӧѡ�õ��ǣ�������| A��CH3CH3��Cl |
| B��CH2=CH2��Cl2 |
| C��CH2=CH2��HCl |
| D��CH3CH3��HCl |
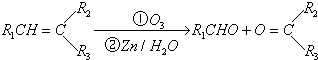

 ��һ�����ܱ�������ͨ��A��B�������壬��һ�������·�����Ӧ��2A��g��+B��g��?2C��g������H��0�����ﵽƽ����������������䣬ֻ�ı�����X������Ӧ��Y�ĸı�һ������ͼ�����ߵ��ǣ�������
��һ�����ܱ�������ͨ��A��B�������壬��һ�������·�����Ӧ��2A��g��+B��g��?2C��g������H��0�����ﵽƽ����������������䣬ֻ�ı�����X������Ӧ��Y�ĸı�һ������ͼ�����ߵ��ǣ�������
