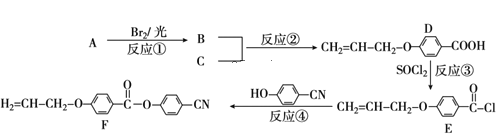
��Ŀ����
����Ŀ����Ԫ�����ڱ��У���ϡ�������⼸������Ԫ�ض��������γ��⻯�
(1)�������ڹ������⻯���ҵ���ð����ʹ��������ͭ��Cu(NH3)2]Ac�Ļ��Һ������CO����������Ӽ�дΪAc-������Ӧ����ʽΪ����Cu(NH3)2]Ac+CO+NH3=[Cu(NH3)3CO]Ac
�ٰ�ˮ��Һ�и�Ԫ��ԭ�ӵĵ�һ�����ܴӴ�С����˳��Ϊ___________��
�ڴ������(CH3COOH)�е�����̼ԭ�ӵ��ӻ���ʽ�ֱ���_________________��
��������[Cu(NH3)3CO]Ac��������ѧ��������_________������ţ���
A�����Ӽ�b��������c�����ۼ�d����λ��
(2)ij���ӻ�����XY2�������ṹ��ͼ��ʾ������6��Yԭ��������1��6��ע��

��֪1��2��3��4��Yԭ���ھ����ϡ������ϣ���5��6��Yԭ�Ӿ��ھ���_________������桱���ڲ�������
�ڸ���������Ϣ������֪��XY2������۷е�______���>������=����<������̬�����۷е㡣
�����þ����ı߳�Ϊanm���ܶ�Ϊ��g/cm3��XY2��Ħ������ΪMg/mol�����ӵ�����Ϊ____��
���𰸡�N��O��H sp3 sp2 acd �ڲ� > ![]()
��������
(1)��ͬ���ڣ���һ�����ܾ�����������ƣ�����VA����ڵ�VIA�壻��CH3COOH�еĵ�һ��̼ԭ���γ�4�����ۼ����ڶ���̼ԭ���γ�3�����ۼ�����������[Cu(NH3)3CO]Ac��Ac����[Cu(NH3)3CO]2+�γ����Ӽ���NH3���й��ۼ���[Cu(NH3)3CO]2+������λ����
(2)��X���Ӹ���Ϊ2�������ݻ�ѧʽXY2�����Y���Ӹ���Ϊ4�����������ļ��������ȷ����������Y��λ�ã��ڸ���������Ϣ������֪��XY2�����Ӿ��壬��̬���Ƿ��Ӿ��壻�۸þ���X���Ӹ���Ϊ2����Y���Ӹ���Ϊ4��������![]() ������ϵʽ���㰢���ӵ�������
������ϵʽ���㰢���ӵ�������
(1)�ٰ�ˮ��Һ��Ԫ��ΪH��N��Oԭ�ӵĵ�һ�����ܴӴ�С����˳��ΪN��O��H���ʴ�Ϊ��N��O��H��
�ڴ������(CH3COOH)�еĵ�һ��̼ԭ���γ�4�����ۼ�����4���������ӻ���ʽΪsp3���ڶ���̼ԭ���γ�3�����ۼ�����3��������û��δ�ɶԵ��ӣ��ӻ���ʽΪsp2���ʴ�Ϊ��sp3��sp2��
��������[Cu(NH3)3CO]Ac��Ac����[Cu(NH3)3CO]2+�γ����Ӽ���NH3���й��ۼ���[Cu(NH3)3CO]2+������λ���������������������ѧ��������abd���ʴ�Ϊ��abd��
(2)��X���Ӹ���Ϊ2�������ݻ�ѧʽXY2�����Y���Ӹ���Ϊ4������֪1��2��3��4��Yԭ���ھ����ϡ������ϣ�����2��������2��Y������5��6�ţ����5��6��Yԭ�Ӿ��ھ����ڲ����ʴ�Ϊ���ڲ���
�ڸ���������Ϣ������֪��XY2�����Ӿ��壬��̬���Ƿ��Ӿ��壬���XY2������۷е㣾��̬�����۷е㣻�ʴ�Ϊ������
�����þ���X���Ӹ���Ϊ2����Y���Ӹ���Ϊ4�������� �����ӵ�����Ϊ
�����ӵ�����Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��