��Ŀ����
1��ǰ�й���ѧԺԺ��¬�����뷨����ô��ѧ��Gignere������������أ�H2NCONH2����H2O2�γɻ�����H2NCONH2•H2O2������ʹH2O2�ȶ�������������ṹҲû�з����ı䣬�õ��˿ɹ�����ʵ��ĵ����壮��֪H2O2�ĽṹʽΪH-O-O-H������˵���в���ȷ���ǣ�������| A�� | H2NCONH2��H2O2��ͨ�������ϵ� | B�� | H2O2������ֻ���Ҽ��������м� | ||
| C�� | H2O2�������������л�ԭ�� | D�� | H2NCONH2•H2O2�������ӻ����� |
���� A��H2O2�Ľṹû�з����ı䣻
B������H2O2�ĽṹʽΪH-O-O-H��֪��H2O2�Ľṹ��ֻ�е�����
C��H2O2����Ԫ�صĻ��ϼ�Ϊ-1�ۣ�������Ԫ�ص��м��̬��
D���ɷǽ���Ԫ���γɵĻ�����������Ϊ���ۻ����
��� �⣺A������ ��H2NCONH2����H2O2�γɼӺ���H2NCONH2•H2O2������ʹH2O2�ȶ����������ҽṹҲû�з����ı䣬��˵����ͨ��������ӵģ���A��ȷ��
B������H2O2�ĽṹʽΪH-O-O-H��֪��H2O2�Ľṹ��ֻ�е�������ֻ��ֻ���Ҽ��������м�����B��ȷ��
C��H2O2����Ԫ�صĻ��ϼ�Ϊ-1�ۣ�������Ԫ�ص��м��̬���������������л�ԭ�ԣ���C��ȷ��
D����������⣬�ɷǽ���Ԫ���γɵĻ�����������Ϊ���ۻ������H2NCONH2•H2O2Ϊ���ۻ������D����
��ѡD��
���� ���⿼�������ͻ�����Ľṹ�����ʣ����׳�������A��Ӧע���������˫��ˮ�ĽṹҲû�з����ı䣬��˵����ͨ��������ӵģ�

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
11������ʵ��������ʵ�ֵ��ǣ�������


| A�� | ��25 mL��ʽ�ζ�����ȡ20.00 mL KMnO4��Һ | |
| B�� | ͼ1�ɱ�ʾ0.1000mol•L-1NaOH��Һ�ζ�20.00mL 0.1000mol•L-1CH3COOH��Һ���õ��ĵζ����� | |
| C�� | ������ʵ��ʱ��ǿ�������Һ�ĵ�������һ�������������Һ�ĵ�������ǿ | |
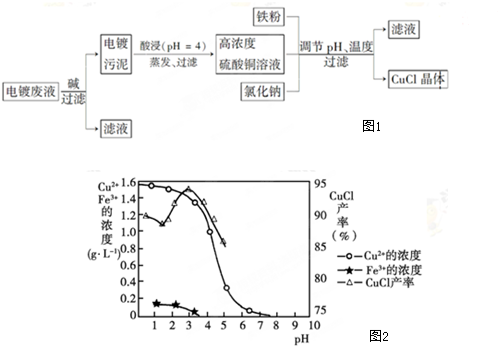
| D�� | ����������ͬ�£�ͼ2��̽��Fe3+��Cu2+��H2O2�ֽⷴӦ�Ĵ�Ч�� |
12����ͼ��a��b��c��d��Ϊʯī�缫������˵����ȷ���ǣ�������


| A�� | ���ձ�����Һ��pH�������ձ�����Һ��pH��С | |
| B�� | ���ձ���a�缫����������Ӧ���缫��ӦʽΪ��4OH--4e-�TO2��+2H2O | |
| C�� | ���һ��ʱ���b���������ӣ�c�������μӷ�̪��Һ��� | |
| D�� | C�����������Ӵ�d�缫ͨ����Һ����c�缫 |
9������˵��������ǣ�������
| A�� | ������и�Ԫ�ص������ٷֺ�������ͬ�������DZض���ͬϵ�� | |
| B�� | ��Է���������ͬ���ṹ��ͬ������һ����ͬ���칹�� | |
| C�� | �����ʵĺ������ͼ���Ի�÷����к��л�ѧ��������ŵ���Ϣ | |
| D�� | �Ӻ˴Ź�������ͼ������֪���л�������м��ֲ�ͬ���͵���ԭ�Ӽ����ǵ���Ŀ |
16�������ܼ����Ų���ȷ���ǣ�������
| A�� | 1s | B�� | 2d | C�� | 3p | D�� | 4f |
6������������R3+������28�����ӣ���������Ϊ70������ڵ��������ǣ�������
| A�� | 42 | B�� | 39 | C�� | 31 | D�� | 28 |
10�� �Ƚ��ĸ������ܶȶ��ε�ض���һ���綯�����ķ�չ�Ϳ�������Դ�������Ч���þ���������Ҫ�����ã�����Al-Mn2O4���ε����һ�����͵�أ���Al3+��Al2Cl7��${AlCl}_{4}^{-}$��ɵ�����Һ��Ϊ�õ�صĵ��Һ����ؽṹ��ͼ��ʾ���ŵ�ʱ���ܷ�ӦʽΪAl+Mn2O4�TAlMn2O4������˵����ȷ���ǣ�������
�Ƚ��ĸ������ܶȶ��ε�ض���һ���綯�����ķ�չ�Ϳ�������Դ�������Ч���þ���������Ҫ�����ã�����Al-Mn2O4���ε����һ�����͵�أ���Al3+��Al2Cl7��${AlCl}_{4}^{-}$��ɵ�����Һ��Ϊ�õ�صĵ��Һ����ؽṹ��ͼ��ʾ���ŵ�ʱ���ܷ�ӦʽΪAl+Mn2O4�TAlMn2O4������˵����ȷ���ǣ�������
 �Ƚ��ĸ������ܶȶ��ε�ض���һ���綯�����ķ�չ�Ϳ�������Դ�������Ч���þ���������Ҫ�����ã�����Al-Mn2O4���ε����һ�����͵�أ���Al3+��Al2Cl7��${AlCl}_{4}^{-}$��ɵ�����Һ��Ϊ�õ�صĵ��Һ����ؽṹ��ͼ��ʾ���ŵ�ʱ���ܷ�ӦʽΪAl+Mn2O4�TAlMn2O4������˵����ȷ���ǣ�������
�Ƚ��ĸ������ܶȶ��ε�ض���һ���綯�����ķ�չ�Ϳ�������Դ�������Ч���þ���������Ҫ�����ã�����Al-Mn2O4���ε����һ�����͵�أ���Al3+��Al2Cl7��${AlCl}_{4}^{-}$��ɵ�����Һ��Ϊ�õ�صĵ��Һ����ؽṹ��ͼ��ʾ���ŵ�ʱ���ܷ�ӦʽΪAl+Mn2O4�TAlMn2O4������˵����ȷ���ǣ�������| A�� | �ŵ�ʱ�������ĵ�ط�ӦʽΪAlMn2O4-3e-�TMn2O4+Al3+ | |
| B�� | �ŵ�ʱ��Al3+���ƶ� | |
| C�� | ���ʱ��Mn2O4�����Դ�ĸ������� | |
| D�� | ���ʱ��Al�缫�������� |