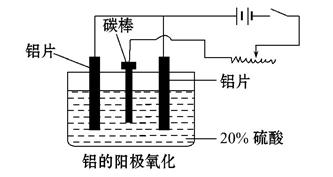
��Ŀ����
��ҵ����ij����(����Cu2O��Al2O3��Fe2O3��SiO2)��ȡͭ�IJ����������£�
��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ__________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)�����õ�ⷨ���д�ͭ����ʱ������������ȷ����________(�����)��
a������ȫ��ת��Ϊ��ѧ��
b����ͭ�ӵ�Դ����������������Ӧ
c����ͭ��������������Һ��Cu2��Ũ�ȼ�С
d����ͭ����ʱͨ���ĵ�������������ͭ������ȷ����ϵ
�ڴ�Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)
(1)���ˡ�����
(2)Fe2����2Fe3����Cu=2Fe2����Cu2�������軯����Һ��������ˮ(����������������)
(3)2Al��Fe2O3 Al2O3��2Fe
Al2O3��2Fe
(4)11
(5)��bc���ڽ�Ũ����������ˮϡ�ͣ����Ƶõ���Ʒ������ϡ�����ַ�Ӧ
����

 ѧҵ����һ��һ��ϵ�д�
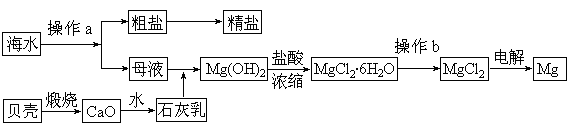
ѧҵ����һ��һ��ϵ�д�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���֪��ˮ��ȡþ����Ҫ�������£�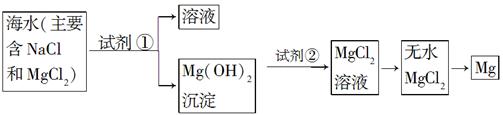
��1�����ڼ����Լ������������������¼��ֲ�ͬ������������������⡣
| ���� | �Ƿ���ȷ | �������� |
| ����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
| ����2�����¼���������ˮ���ټ�������� | ������ | ������ |
| ����Ϊ����������������ǣ����������ģ� | ||
��һ��_______________________________________________��
������_______________________________________________��
������______________________________________________��
���ģ�______________________________________________��
��2����ͼ�м�����Լ���Ӧ����________���ѧʽ����������Լ�����________���ѧʽ������ҵ������ˮMgCl2��ȡþ�Ļ�ѧ����ʽΪ________________________________________��
���ȷ�Ӧ������ұ�����۵�Ľ������ɼ���Ϊ������ijЩ�����������ڸ��������·����ķ�Ӧ��ijѧϰС������ȷ�Ӧ����Al��Fe2O3��ӦΪ����ʵ������о���
�������ݵõ�Al��Al2O3��Fe��Fe2O3���۵㡢�е��������±���ʾ��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565[ |
| �е�/�� | 2467 | 2980 | 2750 | �� |
�Իش��������⣺
��1�������ȷ�Ӧ�н��������ֳ� �ԣ����������ԭ���������ж����н�������һ�����������ȷ�Ӧ��ȡ ��������ţ�
��Fe����Cr����������V����������Ca����Mn
��2��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ�����������Ӧ�������������ֽ����������һ����ʵ�鷽��֤�����������к��н���������ʵ�������õ��Լ�Ϊ ���ɹ۲쵽��ʵ�������� ��
��3����һͬѧ�Ʋ����ȷ�Ӧ�õ����������л�����Fe2O3������������·�������֤��
ȡһ���������Ͷ�뵽����ϡ�����У���Ӧ��Ļ��Һ�еμ����ʼ���Һ���۲���Һ��ɫδ��Ѫ��ɫ������֤���������в�����Fe2O3����
�����ʼ��� ���ѧʽ����
�ڸ�ͬѧ��ʵ�鷽���Ƿ������ ���������������������
���ɣ�
 8Cu��4FeO��2Fe2O3��16SO2
8Cu��4FeO��2Fe2O3��16SO2
 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��