��Ŀ����
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���֪��ˮ��ȡþ����Ҫ�������£�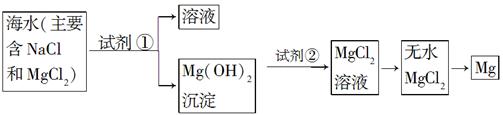
��1�����ڼ����Լ������������������¼��ֲ�ͬ������������������⡣
| ���� | �Ƿ���ȷ | �������� |
| ����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
| ����2�����¼���������ˮ���ټ�������� | ������ | ������ |
| ����Ϊ����������������ǣ����������ģ� | ||
��һ��_______________________________________________��
������_______________________________________________��
������______________________________________________��
���ģ�______________________________________________��
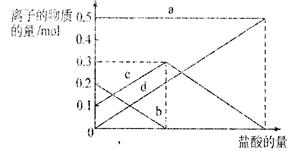
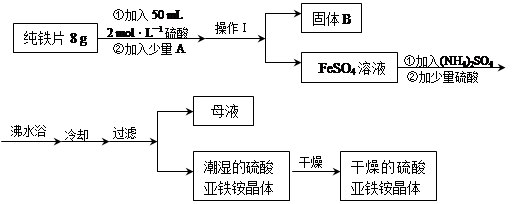
��2����ͼ�м�����Լ���Ӧ����________���ѧʽ����������Լ�����________���ѧʽ������ҵ������ˮMgCl2��ȡþ�Ļ�ѧ����ʽΪ________________________________________��
��1����һ����ˮ��þ����Ũ��С������������������
����������ȷ
��������Դ���Ĵ�����
���ģ���̲ɹ�κ�õ��Ŀ�±ˮ�У����������
��2��Ca��OH��2��HCl��MgCl2�����ڣ�  Mg��Cl2��
Mg��Cl2��
����

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ
����ʯ��һ�ָ�þ�����ο�����ܳƣ�������ɫ������������Ƥ������������ʯ���Կ�����MgO��FeO��Fe2O3��Al2O3��SiO2��ɡ���ҵ��������ʯ��ȡ��ʽ̼��þ��Ʒ���������£�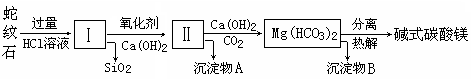
��1������ʯ�������ܽ����Һ�����Mg2����Al3+�⣬�����еĽ���������________��
��2������м����������������� ������Ca(OH)2ʱ��
��Ҫ������ҺpH��7��8֮��(�й��������������pH���±�)��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
��pH��8���ܻᵼ�� �ܽ⡢________������
��3��������A����Ϊ��ȡ��ɫ���ϵ�ԭ�ϣ����������A�м��� ��Ȼ
����ˡ�ϴ�ӡ�_________����дʵ���������)�����ɻ�ú�ɫ���ϣ�ʵ�ַ�����ۺ����á�
��4������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������________(��д���ʻ�ѧʽ)��
��5������Ʒ�Ļ�ѧʽ��aMgCO3��bMg(OH)2��cH2O��ʾ���ֳ�ȡ18.2 g��Ʒ��ʹ֮��ȫ�ֽ⣬�ռ���3.36L CO2����״���£���8.0 g MgO��ͨ������ȷ����Ʒ�Ļ�ѧʽ�У�a��________��b��________��c��________��

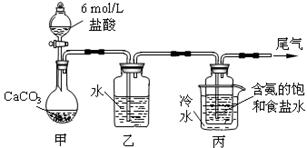
 ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ
ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ