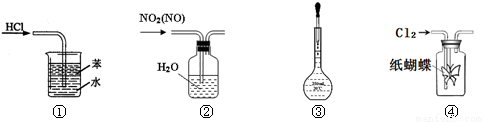
��Ŀ����
(14��)Na2S2O3��5H2O�׳ơ�������������������������Ӱ���ͻ�ԭ��������ɫ������ˮ�ľ��壬�������Ҵ�����20��C��70��Cʱ���ܽ�ȷֱ�Ϊ60.0g��212g�� Na2S2O3��5H2O��40��45��C�ۻ���48��C�ֽ⡣��֪Na2S2O3��ϡ��Һ��BaCl2��Һ����������ɡ�������Na2S2O3��5H2O��ʵ�����Ʊ����������ʵ�顣
�Ʊ������ķ�Ӧԭ����Na2SO3+S Na2S2O3
Na2S2O3
�Ʊ����������̣�
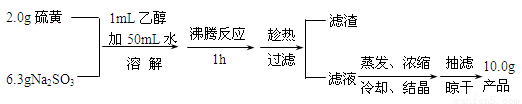
��1��ʵ�鿪ʼʱ��1mL�Ҵ���ʪ��۵������� ������ĸ����
A��������������������Ƶij�ֽӴ�
B����ֹ���������ܽ�
C��������Һ��pH
D����߲�Ʒ�Ĵ���
��2�����ȹ��˵�ԭ���� ��
��3����Һ������ֱ�������ᾧ�Ŀ���ԭ���� ��
��4�����˹�������Ҫϴ�Ӳ�Ʒ���壬����Һ�����ʺϵ��� ������ĸ����
A����ˮ�Ҵ� B������NaCl��Һ C��ˮ D����Һ
��5��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4���뽫����ʵ�鷽�����������������Լ���ϡHNO3��ϡH2SO4��ϡHCl��BaCl2��Һ������ˮ��ѡ��
��ȡ������Ʒ���ϡ��Һ�� �����ɰ�ɫ������
�� ��������δ��ȫ�ܽ⣬���д̼�����ζ������������ȷ����Ʒ�к���Na2SO3��Na2SO4���������Ƶõ�Na2S2O3��5H2O�ֲ�Ʒ��ͨ�� �����ᴿ��
17.��14�֣���1��A��2�֣���2����ֹ�¶Ƚ��Ͷ�ʹNa2S2O3��5H2O����������2�֣�
��3��ֱ�������ᾧ��ʹNa2S2O3��5H2O�ۻ��ֽ⣨2�֣�
��4��A��2�֣���5���ٵμ�����BaCl2��Һ��2�֣�
�ڹ��ˣ�������ˮϴ��������������м�������ϡ���ᣨ2�֣���6���ؽᾧ��2�֣�
��������
�����������1������S������ˮ�����ھƾ���ʵ�鿪ʼʱ��lmL�Ҵ���ʪ��ۣ�������������������ǵij�ֽӴ�����Ϊ��A����2�����¶Ƚ��ͣ�Na2S2O3���ܽ�Ȼ��С������Na2S2O3�ľ������������õ���Na2S2O3���٣���Ϊ����ֹ�¶Ƚ��Ͷ�ʹNa2S2O3������������3������Na2S2O3 40��45���ۻ���48��ֽ⣬��ֱ�������ᾧ����ʹ�����ڻ����ֽ⣬�ò���Na2S2O3?5H2O����Ϊ��ֱ�������ᾧ��ʹNa2S2O3?5H2O�ۻ��ֽ⣻��4������Na2S2O3������ˮ�������ڴ���ѡ����ˮ�Ҵ����Խ�����������Ƶ���ʧ����Ϊ��A��
��5��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4�����岽���Ǣ�ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ�����ɰ�ɫ���������ˣ�������ˮϴ��������������м�������ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ������������ȷ����Ʒ�к���Na2SO3��Na2SO4����6��ֱ�������ᾧ��ʹNa2S2O3?5H2O�ۻ��ֽ⣬�������Ƶõ�Na2S2O3��5H2O�ֲ�Ʒ��ͨ���ؽᾧ�ķ����ᴿ��
���㣺����Na2S2O3��5H2O��ʵ�����Ʊ����������ʵ�顣

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д����л�ѧ������ȷ����
A��HClO�ĵ���ʽ�� |
B��������Ϊ10����ԭ�ӣ� |
C���������Ľṹ��ʽ�� |
D��CH4���ӵ����ģ�ͣ� |
��˹ƥ�֣�����ʽΪC9H8O4����������֪���θ�ðҩ�����н�����ʹ���á� ����Ħ��������
| A��148g | B��148g/mol | C��180g/mol | D��146g |
 Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2+]���¶�T�Ĺ�ϵ��ͼ��ʾ��
Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2+]���¶�T�Ĺ�ϵ��ͼ��ʾ��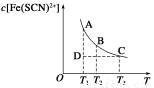
 Fe(SCN)2��(aq) ��H��0
Fe(SCN)2��(aq) ��H��0