��Ŀ����
����Ŀ����I���о������к������Ҫ�� SO2 �� H2S����ת��������Ҫ���塣
��1����ҵ�ϲ��ø����ȷֽ�H2S�ķ�����ȡH2����Ĥ��Ӧ���з���H2�������ķ�ӦΪ�� 2H2S(g) ![]() 2H2(g)��S2(g) ��H
2H2(g)��S2(g) ��H
��֪����H2S(g) ![]() H2(g)��S(g) ��H1�� ��2S(g)
H2(g)��S(g) ��H1�� ��2S(g) ![]() S2(g) ��H2��
S2(g) ��H2��
�� ��H��________________(�ú� ��H1����H2��ʽ�ӱ�ʾ)��
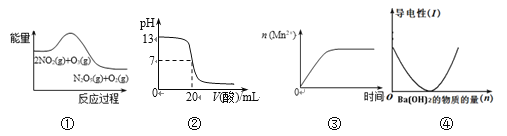
��2�������е�����ɽ������� H2S ��������Ӧ������ SO42-��������Ӧ�������仯ʾ��ͼ���£�

1mol H2S(g)ȫ��������SO42-(aq)���Ȼ�ѧ����ʽΪ________________��
��II��100��ʱ����1L���º��ݵ��ܱ������У�ͨ��0.1mol N2O4��������Ӧ��N2O4(g) ![]() 2NO2(g) ��H����57.0kJ��mol��1��NO2��N2O4��Ũ����ʱ��仯�����ͼ��ʾ��
2NO2(g) ��H����57.0kJ��mol��1��NO2��N2O4��Ũ����ʱ��仯�����ͼ��ʾ��
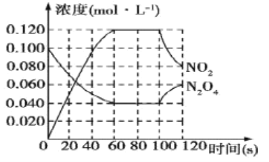
��3����0~60s�ڣ���N2O4��ʾ��ƽ����Ӧ����Ϊ__________mol��L��1��s��1��
��4������ͼ���й����ݣ�����100��ʱ�÷�Ӧ��ƽ�ⳣ��K1��__________���������������䣬�����¶���120�棬�ﵽ��ƽ��ʱ��ƽ�ⳣ����K2����K1_____K2(�>������<������)��
��III�����ݻ�Ϊ2L���ܱ�������ͨ��һ������CO��H2O��������Ӧ��CO(g)��H2O(g) ![]() H2(g)��CO2(g)��
H2(g)��CO2(g)��
��5������˵������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ���____(����ĸ���)��
A.������CO��H2O��CO2��H2��Ũ��֮��Ϊ1��1��1��1
B.CO������������H2�������������
C.������ѹǿ���ֲ���
D.���������ܶȱ��ֲ���
��6�����������������䣺��VL�ܱ�������ͨ��10molCO��10molH2O(g)����������Ӧ����T��ﵽƽ�⣬Ȼ���ٳ�ȥˮ����(��ˮ����ʱ�������ɷֵ����ʵ�������)�����������ȼ�գ���÷ų�������Ϊ2842kJ(��֪CO��ȼ����Ϊ283kJ��mol��1��H2��ȼ����Ϊ286kJ��mol��1)����T��ƽ�ⳣ��K��______������ȷ��С�������λ��
���𰸡�2��H1+ ��H2 H2S(g)+2O2(g) =SO42-(aq)+2H+(aq) ��H =��806.39 kJ��mol-1 1��10-3 0.36 < B 0.44
��������
��1�����ø�˹���ɼ��㣻
��2������ͼ��д���������Ȼ�ѧ����ʽ���ٸ��ݸ�˹���ɼ��ã�
��3������v=![]() ���㣻
���㣻
��4������ƽ�ⳣ���ı���ʽ��ͼ����㣻��Ϊ�÷�Ӧ���ʱ��H>0�������¶ȣ�k����
��5������ƽ������淴Ӧ������ȣ��������ڷ����ı������
��6�����ݷų������������CO�����������ʵ������ڼ���ƽ�ⳣ����
��1����֪����H2S(g) ![]() H2(g)��S(g) ��H1�� ��2S(g)
H2(g)��S(g) ��H1�� ��2S(g) ![]() S2(g) ��H2�����ݸ�˹���ɢ١�2+�ڵ�2H2S(g)
S2(g) ��H2�����ݸ�˹���ɢ١�2+�ڵ�2H2S(g) ![]() 2H2(g)��S2(g) ��H��2��H1+ ��H2��
2H2(g)��S2(g) ��H��2��H1+ ��H2��
�𰸣�2��H1+ ��H2��
��2����ͼ��֪����һ���Ȼ�ѧ��ӦΪ��H2S��g��+0.5O2��g��=S��s��+H2O��g����H=��221.19 kJmol-1���ڶ�����ӦΪ��S��s��+1.5O2��g��+H2O��g��=2H+��aq��+SO42-��aq������H=-585.20 kJmol-1�����ݸ�˹���ɣ���һ����ڶ�������ʽ��ӵã�H2S(g)+2O2(g) =SO42-(aq)+2H+(aq) ��H =��806.39 kJ��mol-1��
�𰸣�H2S(g)+2O2(g) =SO42-(aq)+2H+(aq) ��H =��806.39 kJ��mol-1��
��3���ɼ�ͼ��֪����60sʱ��N2O4��Ũ��Ϊ0.04 mol��L-1������N2O4��ʾ��ƽ����Ӧ����Ϊv=(0.1-0.04) mol��L-1��60s=1��10-3 mol��L-1��s-1��
�𰸣�1��10-3��
��4����ͼ��֪�ڷ�Ӧ��60sʱ����Ӧ����������Ũ�ȱ��ֲ��䣬���Դ�ʱ��Ӧ��ƽ�⣬ƽ�ⳣ��K1=![]() =
=![]() =0.36mol.L-1.s-1����Ϊ�÷�Ӧ���ʱ��H>0�������Ǹ����ȷ�Ӧ�����������¶ȣ�ƽ�������ƶ���ƽ�ⳣ������k1<k2��
=0.36mol.L-1.s-1����Ϊ�÷�Ӧ���ʱ��H>0�������Ǹ����ȷ�Ӧ�����������¶ȣ�ƽ�������ƶ���ƽ�ⳣ������k1<k2��
�𰸣�0.36��<��
��5��A.������CO��H2O��CO2��H2��Ũ��֮��Ϊ1��1��1��1ʱ����Ӧ��һ���ﵽ��ƽ�⣬��A����
B.CO���������ʴ�������Ӧ���ʣ�H2���������ʴ����淴Ӧ���ʣ��������ʱȵ��ڻ�ѧ������֮�ȣ���˷�Ӧ�ﵽ��ƽ�⣬��B��ȷ��
C.��Ϊ��Ӧǰ������������ʵ���ʼ�ղ��䣬��������ѹǿ��Ӧ��ʼ�ղ��䣬�����ж��Ƿ�ﵽƽ�⣬��C����
D.��Ӧ�����У����������ܶ�ʼ�ձ��ֲ��䣬�����ж��Ƿ�ﵽƽ�⣬��D����
�𰸣�B��
��6�����ݷ���ʽ��ϵ�����Կ��������������������Ϊxmol����һ����̼ʣ�ࣨ10-x��mol������ȼ�շų��������г����̣�286x+283��10-x��=2842�����x=4�����Դﵽƽ��ʱ��һ����̼��ˮ���������ʵ����ֱ�Ϊ6mol��������������̼�����ʵ����ֱ�Ϊ4mol�������ڷ�Ӧǰ�����廯ѧ��������ȣ���ƽ�ⳣ���ı���ʽ�У��������������Լȥ��k=![]() =
=![]() =0.44��
=0.44��
�𰸣�0.44��

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д�