��Ŀ����
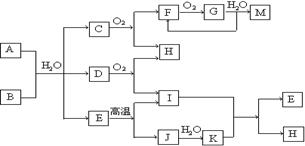
��֪AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ�������³�ѹ��C��D��F��G��I������̬�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ����ش��������⣺

��1��д��B�ĵ���ʽ________________��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ______________________��
��3���������G���м�ѹ������������������_____________��
��4��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M ______________________��
����A��Һ�м������NaOH��Һ�������� _________________________��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ______________________��
��3���������G���м�ѹ������������������_____________��
��4��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M ______________________��
����A��Һ�м������NaOH��Һ�������� _________________________��
(1) 
(2) NH4HCO3 + CaC2 == CaCO3 + NH3��+C2H2��
(3) ����ɫ������ɫ��dz
(4) �� HCO3- + H+ = CO2�� + H2O���� NH4+ + HCO3- + 2OH- NH3��+ CO32-
NH3��+ CO32-

(2) NH4HCO3 + CaC2 == CaCO3 + NH3��+C2H2��
(3) ����ɫ������ɫ��dz
(4) �� HCO3- + H+ = CO2�� + H2O���� NH4+ + HCO3- + 2OH-
 NH3��+ CO32-
NH3��+ CO32- 
��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
�����Ŀ


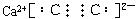


 G���м�ѹ������������������ ��
G���м�ѹ������������������ �� ��Һ�������� ��
��Һ�������� ��