��Ŀ����
��֪AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ����C��D��F��G��I�ڳ��³�ѹ�¾�����̬����Dȼ��ʱ��������Ȳ�泣�������ӻ��и�����������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ����(��������)������ȥ��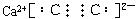
(1)д��B�ĵ���ʽ___________��D�Ľṹ��ʽ___________��
(2)д��A��B��Ӧ�Ļ�ѧ����ʽ____________________________________��
(3)��I����ͨ�����б���C�����NaCl��Һ���Ǻ����Ƽ��һ���ؼ���Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
(4)д�����з�Ӧ�����ӷ���ʽ��
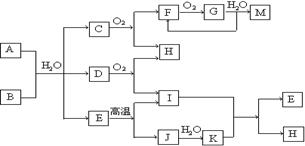
����A��Һ�м���M_________________________________��
����A��Һ�м������NaOH��Һ��������________________________��
����֪Fe3O4������M��Һ������F���壬�����ӷ���ʽΪ_______________________��
(1) ![]() CH��CH
CH��CH
(2)CaC2+NH4HCO3![]() NH3��+C2H2��+CaCO3(���߷ֲ�д)
NH3��+C2H2��+CaCO3(���߷ֲ�д)
(3)NH3+CO2+NaCl+H2O![]() NH4Cl+NaHCO3��
NH4Cl+NaHCO3��
(4)��![]() +H+
+H+![]() H2O+CO2��
H2O+CO2��
��![]() +
+![]() +2OH-
+2OH-![]() NH3��+2H2O+
NH3��+2H2O+![]()
��3Fe3O4+28H++![]()
![]() 9Fe3++NO��+14H2O
9Fe3++NO��+14H2O
����������ͼ�ƶ��⡣ͻ�ƿ��ڡ�Dȼ�ղ�������Ȳ��ɺ��ӻ��и����������֪DΪCH��CH������ת����ϵ����ѧ֪ʶ�����ƶϸ����ʷֱ�Ϊ��A.NH4HCO3��B.CaC2��C.NH3��E.CaCO3��F.NO��G.NO2��H.H2O��I.CO2��J.CaO��K.Ca(OH)2��M.HNO3������ӭ�ж��⡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�



 G���м�ѹ������������������ ��
G���м�ѹ������������������ �� ��Һ�������� ��
��Һ�������� ��