��Ŀ����
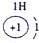
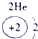
��15�֣��±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
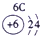
| �� ���� | IA | | 0 | |||||
| 1 | | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | �� | �� | �� | �� | | | |
�ڡ��ܡ��ݵ����Ӱ뾶�ɴ�С��˳��Ϊ____________________��
��2���٢ڢޢ�����Ԫ������Ӧ����̬�⻯�����ȶ�����________����ȶ�����_____���ѧʽ����
(3)�ڡ��ۿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�����������Ӻ������ӵĸ�����Ϊ_____��
��4���ۡ�����Ԫ������������Ӧˮ�������Ӧ�����ӷ���ʽΪ__________________________��
(5)��ҵ���âٵ��ʴ����������ʣ���Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��6���ܢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ�����ԭ�ӽṹ֪ʶ���� ��������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ��������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���ֱ����Ȼ�þ��Һ���Ȼ�����Һ����ε�������������Һֱ��������
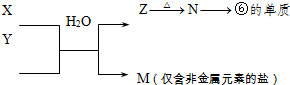
(7)�ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��

X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ____________ _________��
��1��(��1��) Mg Al O, O2- Mg2+ Al3+��2��(��1��) H2O ,SiH4
��3��(1��)2:1��4��(2��) Al��OH��3��OH-��[Al(OH)4]-
��5��(2��) C��SiO2 2CO����Si
2CO����Si
(6)(1+1 +2��)þ ��þԭ�Ӱ뾶����ԭ�Ӱ뾶��ԭ�Ӻ˶���������������������ʧ����������ǿ��bcd��7��(2��) Al3����3NH3��H2O��Al��OH��3����3NH4��
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ








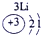
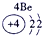
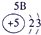
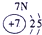
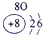
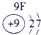
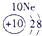
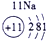





 ��ʾ����
��ʾ����