��Ŀ����
��12�֣����Ե缫���NaCl��Һ��CuSO4��Һ���õ����ֲ���A��B��C��������֮���ת����ϵ����ͼ��ʾ��ͼ�в��뷴Ӧ�����ɵ�ˮ������ȥ������֪���Ƕ�����Ԫ�صĵ��ʣ������ճ������г��õİ�װ���ϡ�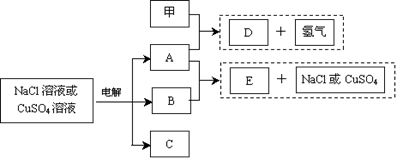
�ش��������⣺
��1����������NaCl��Һ��
�ټ���A��Ӧ�Ļ�ѧ����ʽ������ �������������� ��
��A��B��Ӧ�����ӷ���ʽ������ ������������ ����
�۳����£������100mL 0.1 mol/L NaCl��Һ������������������112mL���壨��״��������������Һ��pHΪ�����������Է�Ӧǰ����Һ������仯����������ˮ��Ӱ�죩��
��2���� ������CuSO4��Һ������ʱ��A��Ũ��Һ����B������Ӧ��
������CuSO4��Һ������ʱ��A��Ũ��Һ����B������Ӧ��
��A��Ũ��Һ��B��Ӧ�����У�A��Ũ����ʱ��仯��ͼ����ȷ�� ��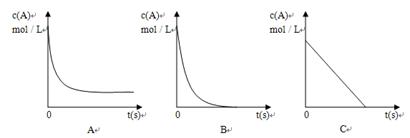
��E�Ļ�ѧʽ�� �����ʱ�����ĵ缫��Ӧʽ������ ��
��1����2Al+ 2NaOH +2H2O = 2NaAlO2 + 3H2��
�� Cl2��2OH��= Cl��+ClO��+H2O�� �� 13
��2����A�� ��SO2 4OH����4e����O2����2H2O
����

��ϰ��ϵ�д�
�����Ŀ
����˼ά�ǻ�ѧ�����г��õ�һ��˼ά�����������й����ӷ���ʽ��������ȷ���ǣ�������
|
��2010?��ƽ��ģ�⣩�±��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ���ǣ�������
|
 ��1�������£���pH=6������ˮ�м���2.3g�����ƣ���ַ�Ӧ���ټ�����ˮϡ�͵�1L��������Һ��pH=
��1�������£���pH=6������ˮ�м���2.3g�����ƣ���ַ�Ӧ���ټ�����ˮϡ�͵�1L��������Һ��pH=