��Ŀ����
18����������Alcoa����ұ���ǵ����ˮAlCl3�����λ����������������������ͼ1�����������ǵ��Al2O3��������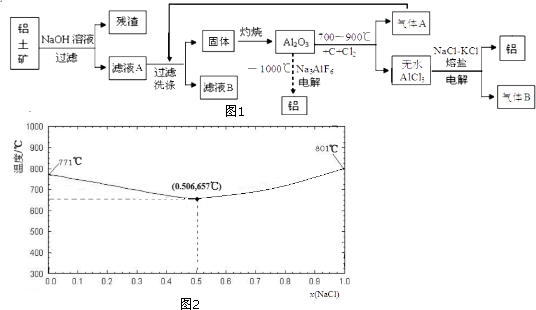
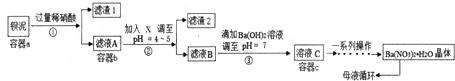
��1����ҺB�м���Ca��OH��2���ѧʽ��ת��ΪNaOH��Һѭ�����ã�������ת���г�����A�⣬����Cl2��ѭ�����ã�
��2��д��Al2O3ת��Ϊ��ˮAlCl3�Ļ�ѧ����ʽ2Al2O3+6Cl2+3C$\frac{\underline{\;700-900��\;}}{\;}$4AlCl3+3CO2����δӷ�Ӧ��Ļ�������з������ˮAlCl3������
��3��NaCl-KCl������ۻ��¶���NaCl�����ʵ����ķ���[x��NaCl��]�仯������ͼ2��
��KCl���۵�Ϊ771�棻���۵����ʱ��NaCl����������Ϊ$\frac{0.506��58.5}{0.506��58.5+0.494��74.5}$���г�����ʽ����������������
����ˮAlCl3��NaCl-KCl�������۽Ⲣ���룬���ⶨ�˻����Һ�к��д���[AlCl4]-�������ӷ���ʽΪAl3++4Cl-?[AlCl4]-���˻����Һ�ĵ���غ�ʽΪn��Na+��+n��K+��+3n��Al3+��=n��Cl-��+n��AlCl4-���������躬������ֻ��Al3+��[AlCl4]-�������˻����Һ������ӦΪAl3++3e-=Al��[AlCl4]-+3e-=Al+4Cl-����ԭ����������Al3+����Na+��K+�������������õ��ӣ��ӵ�������������������ұ�����ŵ���NaCl-KCl������ã���Լ��Դ��
���� �����������̿�֪���������м�����������Һ�����˿ɵ���ҺAΪƫ��������Һ����ҺA��ͨ�������̼�������˿ɵù���������������ҺBΪ̼������Һ��̼������Һ�м����������ƿ������������ƽ���ѭ�����ã���������������������������������������������������̼��������Ӧ��������AΪ������̼����ˮ�Ȼ�������ˮ�Ȼ��������������µ�������BΪ������ͬʱ����������ѭ�����ã��ݴ˴��⣻
��� �⣺�����������̿�֪���������м�����������Һ�����˿ɵ���ҺAΪƫ��������Һ����ҺA��ͨ�������̼�������˿ɵù���������������ҺBΪ̼������Һ��̼������Һ�м����������ƿ������������ƽ���ѭ�����ã���������������������������������������������������̼��������Ӧ��������AΪ������̼����ˮ�Ȼ�������ˮ�Ȼ��������������µ�������BΪ������ͬʱ����������ѭ�����ã�
��1����������ķ�����֪����ҺB�м���Ca��OH��2 ת��ΪNaOH��Һѭ�����ã�������ת���г�����A�⣬��������B��Cl2��ѭ�����ã�
�ʴ�Ϊ��Ca��OH��2��Cl2��
��2����������̼��������Ӧ��������̼����ˮ�Ȼ�������Ӧ����ʽΪ2Al2O3+6Cl2+3C$\frac{\underline{\;700-900��\;}}{\;}$4AlCl3+3CO2���������ķ����ɴӷ�Ӧ��Ļ�������з������ˮAlCl3��
�ʴ�Ϊ��2Al2O3+6Cl2+3C$\frac{\underline{\;700-900��\;}}{\;}$4AlCl3+3CO2��������
��3���ٸ���NaCl-KCl������ۻ��¶���NaCl�����ʵ����ķ���[x��NaCl��]�仯����ͼ��֪��KCl���۵�Ϊ 771�棬���۵����ʱ��NaCl�����ʵ����ķ���Ϊ0.506����ʱNaCl����������Ϊ $\frac{0.506��58.5}{0.506��58.5+0.494��74.5}$��
�ʴ�Ϊ��771�棻$\frac{0.506��58.5}{0.506��58.5+0.494��74.5}$��
����ˮAlCl3��NaCl-KCl�������۽Ⲣ���룬���ⶨ�˻����Һ�к��д���[AlCl4]-�������ӷ���ʽΪ Al3++4Cl-?[AlCl4]-���˻����Һ�ĵ���غ�ʽΪAl3++4Cl-?[AlCl4]-�����˻����Һ��������������ǿ�������ӵõ��ӣ����Է�ӦΪAl3++3e-=Al��[AlCl4]-+3e-=Al+4Cl-����ԭ����������Al3+����Na+��K+�������������õ��ӣ��ӵ�������������������ұ�����ŵ���NaCl-KCl������ã���Լ��Դ��
�ʴ�Ϊ��Al3++4Cl-?[AlCl4]-��n��Na+��+n��K+��+3n��Al3+��=n��Cl-��+n��AlCl4-����Al3++3e-=Al��[AlCl4]-+3e-=Al+4Cl-��������Al3+����Na+��K+�������������õ��ӣ�NaCl-KCl������ã���Լ��Դ��
���� ���⿼���˻������롢�ᴿ�������������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ����ʵ��Ŀ�ļ���Ӧԭ��Ϊ���ؼ���ע�����ջ�ѧʵ��������������������ֿ�����ѧ���ķ�����������������ѧʵ�顢��ѧ����������

| A�� | ��������Ԫ�ص����Ӱ뾶��������С | |
| B�� | ����A��Ԫ�ص��⻯���У��ȶ�����õķе�Ҳ��� | |
| C�� | �ڢ�A��Ԫ�صĽ����Աȵڢ�A��Ԫ�صĽ�����ǿ | |
| D�� | ͬ���ڷǽ���Ԫ���������Ӧˮ��������Դ�����������ǿ |
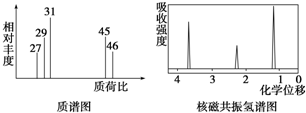
 ç�����������Ϊ�ϳɴ�ƣ��������Ϳ���ҩ�����м�����ܵ����ӣ���ṹ��ʽ��ͼ�����й���ç�����˵����ȷ���ǣ�������
ç�����������Ϊ�ϳɴ�ƣ��������Ϳ���ҩ�����м�����ܵ����ӣ���ṹ��ʽ��ͼ�����й���ç�����˵����ȷ���ǣ�������| A�� | �����к������ֺ��������� | |
| B�� | �ɷ���ȡ�����ӳɼ�������Ӧ | |
| C�� | ��ˮ��Һ���ǻ����Ȼ����ܵ���������� | |
| D�� | ���������Ʒ�Ӧ�ڱ�����ܲ���44.8L���� |
| A�� | ϡ������ | B�� | ����Ԫ�� | C�� | ����Ԫ�� | D�� | �ǽ���Ԫ�� |
| A�� | ���ߵ���������ͬ | B�� | ���ߵ���������ͬ | ||
| C�� | ���ߵĺ����������ͬ | D�� | ����Ϊͬ�������� |