��Ŀ����
5���Ƶõ�̼������Ʒ��������������NaCl�������ⶨ��Ʒ��Na2CO3������������ij̽����ѧϰС��ֱ����������ʵ�鷽������ش������й���������һ����һ����������Ʒ�ܽ����������CaCl2��Һ�������ó������ˣ���������ƣ���ϴ�ӡ���ɡ����������㣮ϴ�ӳ����ľ���������ز�������������������м�����ˮ����û�����ʹˮ��Ȼ���£��ظ�2��3�Σ�
����������һ��������Ʒ���������ᷴӦ������ͼ��ʾװ�òⶨ����CO2�������Ϊ��ȷ���ⶨ�����ȷ�ԣ�B�е���Һ��ò��ñ���NaHCO3��Һ��ͼ1װ����ʵ������a��������Բ����ƿ��

������������ͼ2��ʾװ�����ⶨ������Ʒ��̼���Ƶ���������������̨�����е���ͼ�о�����ȥ����ʵ�鲽�����£�
�ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ�ҵĸ����D������Ϊ33.4g��
��ȷ�Ƶ�6g������Ʒ��������b�У�
�ܴ�Һ©��a����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ��Ƶø����D��������Ϊ35.6g��
��1�����ܢ�������ʵ�����̫�죬��ᵼ�²ⶨ���ƫС���ƫ��ƫС������
��2��װ��A���Լ�XӦѡ��NaOH��Һ��
��3��Eװ�õ������Ƿ�ֹ������CO2��ˮ��������D�У�
��4������ʵ���в�õ��й����ݣ����㴿����ƷNa2CO3����������Ϊ88.3%���������С�����һλ����
���� ����һ�������Һ�����ķ���Ϊ���ˣ�ϴ�ӳ����IJ������ڹ������м�ˮ��û����ʹˮ��Ȼ���£��ظ�����2-3�Σ�
����������һ�����Ļ�������������ᷴӦ��Ȼ������ͼװ�òⶨ������CO2������������������Һ���ⶨ���ɶ�����̼������������м��㣬���ƿ��Һ���DZ���̼�����ƣ�����װ��ͼ������
���������ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ�ҵĸ����D������Ϊ33.4g�������������ն�����̼��������õ����ɶ�����̼�����ȷ������
��ȷ�Ƶ�6g������Ʒ��������b�У�
�ܴ�Һ©��a����������������ϡ���ᣬ�����ٲ�������Ϊֹ��ʹ̼����ȫ����Ӧ��
�ݴ��ɼУ����Թ�A�л���������������Ӱ����ɵ�����ȫ������D��Ȼ��Ƶø����D��������Ϊ35.6g��
��1��ʵ�����̫�죬�������̼������ȫ����ʯ�����գ�
��2��X�����������տ����еĶ�����̼��
��3�������еĶ�����̼��ˮ��������D�л�Ӱ��ʵ�飻
��4������ʵ��ǰ��װ��D�����仯�����ɴ������������Ʒ��̼���Ƶ������������Ʒ��̼���Ƶ�����������
��� �⣺����һ����Ʒ�ܽ����������CaCl2��Һ������̼��Ƴ����������Һ�����ķ���Ϊ���ˣ�ϴ�ӳ����IJ������ز�������������������м�����ˮ����û�����ʹˮ��Ȼ���£��ظ�����2-3�Σ�
�ʴ�Ϊ�����ˣ��ز�������������������м�����ˮ����û�����ʹˮ��Ȼ���£��ظ�2��3�Σ�
����������һ��������Ʒ���������ᷴӦ��Ȼ������ͼװ�òⶨ������CO2������������������Һ���ⶨ���ɶ�����̼������������ڱ���̼��������Һ�ж�����̼����Ӧ���ܽ����С������B�е���Һ��ò��ñ���NaHCO3��Һ����װ��ͼ��֪��ͼװ����ʵ������aΪԲ����ƿ��
�ʴ�Ϊ������NaHCO3��Һ��Բ����ƿ��
����������1����Ӧ�����ʹ���������������̼û����ȫ��Dװ���м�ʯ�����գ����ٹ��������Ҳ��ʹװ���ڲ���������̼���ܱ�Dװ���м�ʯ����ȫ���գ����õĶ�����̼����ƫС�����Բⶨ���ƫС��
�ʴ�Ϊ��ƫС��
��2������������ɰѲ�����װ��B��C�ж�����̼ȫ����D�м�ʯ�����գ���Ϊ�����к��ж�����̼�����Ӧ�ѹ���Ŀ����еĶ�����̼���մ���������װ��AӦ��������������Һ��
�ʴ�Ϊ��NaOH��Һ��
��3�����Dװ��ֱ��������������ͨ��������е�ˮ�Ͷ�����̼��Բⶨ�������Ӱ�죬����װ��E���������Ƿ�ֹ������ˮ�Ͷ�����̼����װ��D�У�
�ʴ�Ϊ����ֹ������CO2��ˮ��������D�У�
��4����Ӧ�зų�������̼���������=85.6g-83.4g=2.2g
��ų�2.2g������̼����̼���Ƶ�����Ϊx
Na2CO3��CO2
106 44
x 2.2g
��� x=5.3g
������ƷNa2CO3����������=$\frac{5.3g}{6g}$��100%��88.3%
�ʴ�Ϊ��88.3%��
���� ���⿼�������ʺ����IJⶨ���������ʵ�̽����ʵ�������������Ҫ��ʵ����̵ķ��������ջ��������ǽ���ؼ�����Ŀ�Ѷ��еȣ��ⶨ������̼����ʱע������еijɷֶ�ʵ���Ӱ�죮

| A�� | ������Zn�缫��������KOH��Һ�������� | |
| B�� | ������ӦʽΪ2FeO42-+10H++6e-=Fe2O3+5H2O | |
| C�� | �õ�طŵ�����е������ҺŨ�Ȳ��� | |
| D�� | ��ع���ʱOH-��Ǩ�� |
| A�� | ���Ϸ�Ӧ | B�� | �ֽⷴӦ | C�� | �û���Ӧ | D�� | ���ֽⷴӦ |
| A�� | ͬϵ�� | B�� | ͬλ�� | ||
| C�� | ͬ�������� | D�� | ͬ�����ʵIJ�ͬ���� |
 �����ӹ�ҵ��NF3�������������ʴ�̼�����ҵ��ͨ����⺬NH4F�ȵ���ˮ����������NF3������ԭ����ͼ��ʾ��
�����ӹ�ҵ��NF3�������������ʴ�̼�����ҵ��ͨ����⺬NH4F�ȵ���ˮ����������NF3������ԭ����ͼ��ʾ��
 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +CO2��
+CO2�� $��_{��}^{NaOH��aq��}$
$��_{��}^{NaOH��aq��}$ +H2O
+H2O $��_{����H+}^{����_{10}%NaOH��Cu}$
$��_{����H+}^{����_{10}%NaOH��Cu}$
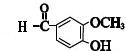 +CH3COCH2COCH3$��_{��}^{NaOH��aq��}$
+CH3COCH2COCH3$��_{��}^{NaOH��aq��}$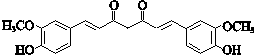 +2H2O��
+2H2O��
