��Ŀ����


(1)д���������ʵĻ�ѧʽ��B___________J__________��
(2)д�����з�Ӧ�����ӷ���ʽ��
��H+E(��Һ)��M________________����I����G______________��?
(3)��ͨ��״���£���1 g C������B������ȼ������H����ʱ�ų�92.3 kJ��������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ____________________��
(2)��H+ +ClO��= HClO��NH3+H2O
 NH3H2O
NH3H2O NH4��+OH-
NH4��+OH- (3)2HCl(g)=H2(g)+Cl2(g) ��H= +184.6 kJ/mol

��ѡ���⣩��19�֣�ͼ������ģ�ͷ��dz��õĿ�ѧ�о�������
I����ͼ���о�����Ԫ�ص��⻯��ķе�仯���ɵ�ͼ��ͬͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ������������ߡ�������a������b������A���Ӧ�ķе���100�棩������Ϊ��ȷ���� �������� ��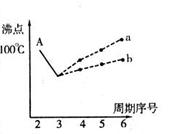
II��������ʹ�ý�������ʷ�����У�������ͭ��������֮�����ֽ����㷺Ӧ�õĽ�����ѧ��Ԥ��Ϊ���ѣ�Ti�����ѱ���Ϊ��δ�����͵Ľ��������Իش��������⣺
��1��22TiԪ�ػ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ ��
��2����Ti�Ļ������У����Գ���+2��+3��+4���ֻ��ϼۣ�������+4�۵�Ti��Ϊ�ȶ���ƫ���ᱵ�����ȶ��Ժã��۵糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�á�ƫ���ᱵ�����о����Ľṹʾ��ͼ����ͼ�������Ļ�ѧʽ�� ��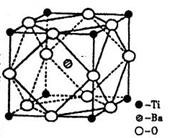
III��������60�껯����һ��ϡ�����廯����Xe[PtF6]���ϳɳ��������ˡ��������ԡ��Ĺ�������ļ����ڣ���ѧ������̺����믵ķ����������ȡ�
��1������Pt�ڲ�ԭ�ӵĶѻ���ʽ��ͭ���ɱ��е�CO2��ͬ����ͼ��������Pt������ʾ��ͼ����˵��Ptԭ���ھ����е�λ�� ��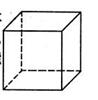
��2��ϡ�����壨뱳��⣩�У�ֻ�н��ص���ܺϳɳ����ֻ����
�����ԭ���� ������ĸ���ţ�
| A��믵ĺ����ȽϷḻ | B��믵����ԭ�������� |
| C���ԭ�Ӱ뾶������С | D���ԭ�Ӱ뾶С���縺�Դ� |






 RH+Na2CO3��
RH+Na2CO3��
 K��
K��