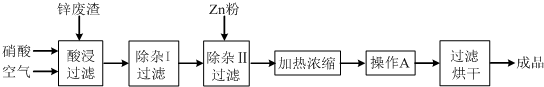
��Ŀ����
����ʯ��������õ�����[KAl��SO4��2?12H2O]���������Ʊ�Al��K2SO4����H2SO4�Ĺ��չ�����ͼ��ʾ��

���������Ļ�ѧ����ʽΪ��4KAl��SO4��2?12H2O+3S�T2K2SO4+2Al2O3+9SO2+48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У��������� ���������뻹ԭ�������ʵ���֮��Ϊ ��
��2����ˮ�������Һ�еõ�K2S04����ķ����� ������K2SO4��KԪ�صļ������������� ��
��3����������Һ����μ���Ba��OH��2��Һ��SO2-4ǡ�ó�����ȫ�����ӷ�Ӧ����ʽΪ ��
��4�����ղ�����SO2�����������ᣮ��֪25�桢l0lkPaʱ��
2SO2��g��+O2��g��?2SO3��g����H1=-197kJ/mol��
H2O��g��?H2O��l����H2=-44kJ/mol��
2SO2��g��+O2��g��+2H2O��g���T2H2SO4��H3=-545kJ/mol��
��SO3��g����H2O��1����Ӧ���Ȼ�ѧ����ʽ�� ��
��5����������������û��������ʧ���������������ղ���K2SO4��Al��H2SO4�����ʵ���֮��Ϊ ��

���������Ļ�ѧ����ʽΪ��4KAl��SO4��2?12H2O+3S�T2K2SO4+2Al2O3+9SO2+48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У���������
��2����ˮ�������Һ�еõ�K2S04����ķ�����
��3����������Һ����μ���Ba��OH��2��Һ��SO2-4ǡ�ó�����ȫ�����ӷ�Ӧ����ʽΪ
��4�����ղ�����SO2�����������ᣮ��֪25�桢l0lkPaʱ��
2SO2��g��+O2��g��?2SO3��g����H1=-197kJ/mol��
H2O��g��?H2O��l����H2=-44kJ/mol��
2SO2��g��+O2��g��+2H2O��g���T2H2SO4��H3=-545kJ/mol��
��SO3��g����H2O��1����Ӧ���Ȼ�ѧ����ʽ��
��5����������������û��������ʧ���������������ղ���K2SO4��Al��H2SO4�����ʵ���֮��Ϊ
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ȼ�ѧ����ʽ
ר�⣺ʵ�������
��������1�����ݻ�ѧ����ʽ��Ԫ�ػ��ϼ۱仯�����жϣ�Ԫ�ػ��ϼ۽��͵�����������Ԫ�ػ��ϼ����ߵ�����ԭ����
��2����ˮ�������Һ�еõ�K2SO4����ķ���������������ܽ�����¶ȱ仯���������������ܼ������ᾧ��������������ɫ��Ӧ����ɫ��
��3������SO42-�����ʵ���Ϊ2mol���ж���ȫ��Ӧ��Ҫ�������ӵ����ʵ�����������д��Ӧ�����ӷ���ʽ��
��4�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
��5��Al2O3���õ������ʣ���������������ȫ����Ӧ�õ����ᣮ
��2����ˮ�������Һ�еõ�K2SO4����ķ���������������ܽ�����¶ȱ仯���������������ܼ������ᾧ��������������ɫ��Ӧ����ɫ��
��3������SO42-�����ʵ���Ϊ2mol���ж���ȫ��Ӧ��Ҫ�������ӵ����ʵ�����������д��Ӧ�����ӷ���ʽ��
��4�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
��5��Al2O3���õ������ʣ���������������ȫ����Ӧ�õ����ᣮ
���
�⣺��1��4KAl��SO4��2?12H2O+3S�T2K2SO4+2Al2O3+9SO2+48H2O��Ӧ��������Ԫ�ػ��ϼ�����Ϊ+4�ۣ��������������Ԫ�ػ��ϼ۴�+6�۱仯Ϊ+4�ۣ���ӡֽ��ԭ�������ʣ��������뻹ԭ�������ʵ���֮��Ϊ6��3=2��1���ʴ�Ϊ��KAl��SO4��2?12H2O��2��1��
��2����ˮ�������Һ�еõ�K2SO4����ķ���������������ܽ�����¶ȱ仯���������������ܼ������ᾧ�������壻��������ɫ��Ӧ����ɫ����Ҫ����ɫ�ܲ����۲죬�ʴ�Ϊ�������ᾧ��������ϴ����˿���û��ոɺ��ò�˿պȡ������Һ�����ƾ��ƻ��������գ�����ɫ�ܲ����۲���ɫ���۲쵽��ɫ��֤���м����ӣ�
��3������SO42-�����ʵ���Ϊ2mol����������Һ�к���2mol SO42-��1molAl3+����������Һ����μ���Ba��OH��2��Һ��SO42-�պó�����ȫʱ����Ҫ2molBa��OH��2���������Ba2+Ϊ2mol��OH-Ϊ4mol������2molBaSO4��1molAl3+��4molOH-��Ӧ����1molAlO2-����Ӧ�����ӷ���ʽΪAl3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O��
�ʴ�Ϊ��Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O��
��4��2SO2��g��+O2��g��?2SO3��g����H1=-197kJ/mol����
H2O��g��?H2O��l����H2=-44kJ/mol����
2SO2��g��+O2��g��+2H2O��g���T2H2SO4��l����H3=-545kJ/mol����
���ݸ�˹���ɢ�-��-2����õ���2SO3��g��+2H2O��l��=2H2SO4��l����H=-260KJ/mol��
����Ӧ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l����H=-130KJ/mol��
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l����H=-130KJ/mol��
��5�����ݷ���ʽ4KAl��SO4��2?12H2O+3S�T2K2SO4+2Al2O3+9SO2+48H2O��Al2O3���õ������ʣ���������������ȫ����Ӧ�õ����ᣮ��֪���ղ���K2SO4��Al��H2SO4�����ʵ���֮��Ϊ2��4��9���ʴ�Ϊ��2��4��9��
��2����ˮ�������Һ�еõ�K2SO4����ķ���������������ܽ�����¶ȱ仯���������������ܼ������ᾧ�������壻��������ɫ��Ӧ����ɫ����Ҫ����ɫ�ܲ����۲죬�ʴ�Ϊ�������ᾧ��������ϴ����˿���û��ոɺ��ò�˿պȡ������Һ�����ƾ��ƻ��������գ�����ɫ�ܲ����۲���ɫ���۲쵽��ɫ��֤���м����ӣ�
��3������SO42-�����ʵ���Ϊ2mol����������Һ�к���2mol SO42-��1molAl3+����������Һ����μ���Ba��OH��2��Һ��SO42-�պó�����ȫʱ����Ҫ2molBa��OH��2���������Ba2+Ϊ2mol��OH-Ϊ4mol������2molBaSO4��1molAl3+��4molOH-��Ӧ����1molAlO2-����Ӧ�����ӷ���ʽΪAl3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O��
�ʴ�Ϊ��Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O��
��4��2SO2��g��+O2��g��?2SO3��g����H1=-197kJ/mol����
H2O��g��?H2O��l����H2=-44kJ/mol����
2SO2��g��+O2��g��+2H2O��g���T2H2SO4��l����H3=-545kJ/mol����
���ݸ�˹���ɢ�-��-2����õ���2SO3��g��+2H2O��l��=2H2SO4��l����H=-260KJ/mol��
����Ӧ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l����H=-130KJ/mol��
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l����H=-130KJ/mol��
��5�����ݷ���ʽ4KAl��SO4��2?12H2O+3S�T2K2SO4+2Al2O3+9SO2+48H2O��Al2O3���õ������ʣ���������������ȫ����Ӧ�õ����ᣮ��֪���ղ���K2SO4��Al��H2SO4�����ʵ���֮��Ϊ2��4��9���ʴ�Ϊ��2��4��9��
���������⿼����������ԭ��Ӧ��������жϣ��Ȼ�ѧ����ʽ��˹���ɵļ��㣬Ԫ���غ�ļ���Ӧ�ã���Ŀ�Ѷ��еȣ�ע�������йص����ӷ���ʽ����д������

��ϰ��ϵ�д�
�����Ŀ
��NA��ʾ�����ӵ�������ֵ�����������в���ȷ���ǣ�������
| A�����³�ѹ�£�3.36L������2.7g����ַ�Ӧת�Ƶĵ�����С��0.3NA |
| B����״���£�5.6L O2��������ʱת�Ƶ�����һ��ΪNA |
| C��0.1molMg�ڿ�������ȫȼ������MgO��Mg3N2��ת�Ƶĵ�����Ϊ0.2NA |
| D��80g CuO��Cu2S�Ļ���ﺬ��ͭԭ����һ��ΪNA |