��Ŀ����
��18mol/L Ũ��������240mL3.0mol/L ϡ�����ʵ�鲽�����£�
�ټ�������Ũ�������� ����ȡһ�������Ũ���� ��ϡ�� ��ת�� ��ϴ�� ���� ��ҡ��
��ش���������
��1��������Һ������ƿ�Ĺ���� ����������ѡ�ã�A.100mL��B.250mL��C.500mL��D.1000mL��������Ũ���������� ��
��2��ʹ������ƿǰ������е�һ�������� ��
��3����������������Ƶ�ϡ����Ũ���к�Ӱ�죿���á�ƫ����ƫС��������Ӱ�족��д��
A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ������� ��
B������ƿ������ϴ�Ӻ������������ˮ ��
�ټ�������Ũ�������� ����ȡһ�������Ũ���� ��ϡ�� ��ת�� ��ϴ�� ���� ��ҡ��
��ش���������
��1��������Һ������ƿ�Ĺ����
��2��ʹ������ƿǰ������е�һ��������
��3����������������Ƶ�ϡ����Ũ���к�Ӱ�죿���á�ƫ����ƫС��������Ӱ�족��д��
A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ�������
B������ƿ������ϴ�Ӻ������������ˮ
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
��������1������240mL��Һ��ʵ������û��240mL������ƿ��������Ҫѡ��250mL������ƿ��������Һϡ���������ʵ����ʵ�������������ҪŨ����������
��2������ƿ����ƿ�������ƹ�������Ҫҡ�ȣ�����ʹ������ƿ������м������ƿ�Ƿ�©ˮ��
��3������c=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��2������ƿ����ƿ�������ƹ�������Ҫҡ�ȣ�����ʹ������ƿ������м������ƿ�Ƿ�©ˮ��
��3������c=
| n |
| V |
���
��1������240mL3.0mol/Lϡ���ᣬ��Ҫѡ�ù����ӽ���250mL����ƿ������B��ȷ����Ҫ18mol/LŨ��������Ϊ��
��0.04167L=41.67mL��
�ʴ�Ϊ��B�� 41.67 mL��
��2��ʹ������ƿ�ĵ�һ������Ϊ�������ƿ�Ƿ�©ˮ��
�ʴ�Ϊ����©��
��3��A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ������У�����Ũ����Ũ��ƫС����ȡ��Ũ��������ʵ���ƫС������c=
�ɵã����Ƶ���Һ��Ũ��ƫС��
�ʴ�Ϊ��ƫС��
B������ƿ������ϴ�Ӻ������������ˮ������ʱ����Ҫ��������ˮ�����Բ�Ӱ�����ƽ����
�ʴ�Ϊ����Ӱ�죮
| 3.0mol/L��0.25L |
| 18mol/L |
�ʴ�Ϊ��B�� 41.67 mL��
��2��ʹ������ƿ�ĵ�һ������Ϊ�������ƿ�Ƿ�©ˮ��
�ʴ�Ϊ����©��
��3��A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ������У�����Ũ����Ũ��ƫС����ȡ��Ũ��������ʵ���ƫС������c=
| n |
| V |
�ʴ�Ϊ��ƫС��
B������ƿ������ϴ�Ӻ������������ˮ������ʱ����Ҫ��������ˮ�����Բ�Ӱ�����ƽ����
�ʴ�Ϊ����Ӱ�죮
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ��������������������е��Ѷȵ����⣬���������ǿ�������߿��������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ���������������Ҫ��ȷ�������ķ����ͼ��ɣ�

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
��֪0.02mol?L-1 CH3COOH��Һ��0.01mol?L-1 NaOH��Һ�Ե������ͺ���Һ�����ԣ���û��Һ����Ũ�ȹ�ϵ��ȷ�ģ�������
| A��c��CH3COO-����c ��Na+�� |
| B��c��Na+��+c��H+��=c��OH-��+c��CH3COOH��+c��CH3COO-�� |
| C��c��CH3COOH����c��CH3COO-�� |
| D��c��CH3COOH��+c��CH3COO-��=0.02mol?L-1 |
���и��������ܴ���������ǣ�������
| A��NaClOˮ��Һ�У�Fe2+��Cl-��Ca2+��H+ |
| B������KSCN�Ժ�ɫ����Һ��K+��Na+��I-��S2- |
| C����ɫ������Һ�У�K+��CH3COO-��HCO3-��MnO4- |
| D��pH=2����Һ�У�NH4+��Na+��Cl-��Cu2+ |
�������ӷ���ʽ��д��ȷ���ǣ�������
| A��Fe2O3��������Fe2O3+6HI=2Fe3++6I-+3H2O |
| B��Na2S��Һ�Լ��ԣ�S2-+2H2O=H2S+2OH- |
| C��CH3COOH��NaOH��Һ��Ӧ��H++2OH-=H2O |
| D��AgNO3��Һ�м��������ˮ��Ag++2NH3?H2O=Ag��NH3��2++2H2O |
��NA��ʾ�����ӵ�����������������ȷ���ǣ�������
| A��46g�Ҵ��к��еĻ�ѧ����Ϊ7NA |
| B��1molOH-��1mol-OH���ǻ����к��е���������Ϊ9NA |
| C��22.4L����������NaOH��Һ��Ӧת�Ƶ�����ΪNA |
| D��0.1mol?L-1FeCl3��Һ�Ƴɽ��壬����Fe��OH��3������һ��С��O.1 NA |
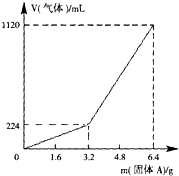 ��һ������Ͼ��ȵ�����������ڸ��������������¹��ȣ���ַ�Ӧ����ȴ�����£��õ�����A��������Ϊm�Ĺ���A���뵽300mL 2mol?L-1������ʹ֮��ȫ�ܽ⣮��������¼������A���������ռ�������������ѻ���ɱ�״�����Ĺ�ϵ��ͼ15��ʾ������������������Һ����ǰ�����������ݳ�����
��һ������Ͼ��ȵ�����������ڸ��������������¹��ȣ���ַ�Ӧ����ȴ�����£��õ�����A��������Ϊm�Ĺ���A���뵽300mL 2mol?L-1������ʹ֮��ȫ�ܽ⣮��������¼������A���������ռ�������������ѻ���ɱ�״�����Ĺ�ϵ��ͼ15��ʾ������������������Һ����ǰ�����������ݳ�����
