��Ŀ����
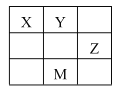
����Ŀ���Ʊ�пӡˢ��·������ϡ���ḯʴп������ķ�Һ�����ð�Һ�������ð�Һ���г�������п�⣬����������ˮ�����Cl����Fe3+����ʵ����������ð�Һ��ȡZnSO47HO�Ĺ�����ͼ��ʾ
��1����ϡ���ḯʴп��ʱ��ԭ����ΪN2O���������뻹ԭ�������ʵ���֮����_________
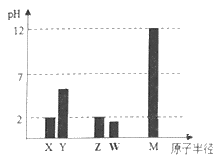
��2��������ٵ�pH��12����Zn(OH) 2�ܽ�����ƫп���ƣ�Zn(OH) 2�ܽ�����ӷ���ʽΪ___________________________
��3����ҺD�г��˺���OH�������⣬�����е���������___________�������ӷ��ţ�
��4������ҺE��pH��4��c��Zn2������2mol��L-1,��Fe3������2.6��10-9mo��L-l�����Fe��OH��3���ܶȻ�����________________��ֻд�����
��5����֪:��Fe��OH��3��s�� Fe3����aq����3OH����aq��H��a kJ mol��L-1
��H2O(l) H����aq����OH����aq��H��b kJ��mol��L-1
���ٵ��ܶȻ�����ΪKsp���ڵ����ӻ�����ΪKw��Fe3������ˮ�ⷴӦ��ƽ�ⳣ��:K��______________���ú�Kw��Ksp�Ĵ���ʽ��ʾ��
���𰸡�1:2 Zn��OH��2: ��2OH�� ��ZnO22�� ��2H2O Cl��,NO3�� 2.6��10-39 K��![]()
��������
��1����ϡ���ḯʴп��ʱ��ԭ����ΪN2O����Ӧ�ķ���ʽΪ4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O�����ݷ���ʽ�ж��������뻹ԭ�������ʵ���֮�ȣ�
��2�����ݷ�Ӧ���������ȷ����Ӧ�����ӷ���ʽ��
��3��������������ԣ�����A��������Һ�е�Cl����NO3�������ӣ�����Һ��Ӧ����Cl����NO3����
��4����ҺE��pH=4���õ��ij���ΪFe��OH��3��ֻ�ܼ���Fe��OH��3���ܶȻ���
��5�����ø�˹���ɼ��㷴Ӧ�ȣ���д��Fe3������ˮ�ⷴӦ���Ȼ�ѧ����ʽ�����â٢�ƽ�ⳣ����ȷ��Fe3������ˮ�ⷴӦ��ƽ�ⳣ����
��1����ϡ���ḯʴп��ʱ��ԭ����ΪN2O����Ӧ�ķ���ʽΪ4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O���ɷ���ʽ��֪��Ӧ��HNO3Ϊ��������ZnΪ��ԭ������4molZn�μӷ�Ӧʱ����2molHNO3����ԭ�����������뻹ԭ�������ʵ���֮��1��2��
��2��Zn��OH��2�ܽ��������ǻ���п���ƣ���Ӧ�����ӷ���ʽΪZn��OH��2+2OH��=[Zn��OH��4]2-��
��3��������������ԣ�����A��������Һ�е�Cl����NO3�������ӣ�����Һ��Ӧ����Cl����NO3����
��4����ҺE��pH=4���õ��ij���ΪFe��OH��3��ֻ�ܼ���Fe��OH��3���ܶȻ�=2.6��10-9����10-10��3=2.6��10-39��
��5��Fe3������ˮ�ⷴӦ�ķ���ʽΪFe3��+3H2O![]() Fe��OH��3+3H����
Fe��OH��3+3H����
��֪��Fe��OH��3��s��![]() Fe3����aq��+3OH����aq����H=aKJ��mol��1
Fe3����aq��+3OH����aq����H=aKJ��mol��1
��H2O��l��![]() H����aq��+OH����aq����H=bKJ��mol��1��
H����aq��+OH����aq����H=bKJ��mol��1��
�����ø�˹���ɽ�����3-�ٿɵ�Fe3����aq��+3H2O��l��![]() Fe��OH��3��aq��+3H����aq����H=��3b-a��KJ��mol��1��
Fe��OH��3��aq��+3H����aq����H=��3b-a��KJ��mol��1��
��Fe3������ˮ�ⷴӦ��ƽ�ⳣ��K=![]() ��
��

����Ŀ��ijͬѧ̽������Na��CO2�ķ�Ӧ��ʵ�����£�
ʵ��I | ʵ��II | |
���� | ����ȼ�Ľ������쵽ʢ��CO2�ļ���ƿ�� | ��ʵ��I�ļ���ƿ��ˮ��ϴ�����ˡ�ȡ��ɫ�������գ�ȡ��Һ�ֱ�μӷ�̪���Ȼ�����Һ |
���� | �ٻ���ʻ�ɫ �ڵײ��к�ɫ���壬ƿ���ϸ��а�ɫ���� | �ٺ�ɫ������ȼ ����Һ��ʹ��̪��Һ��죬�μ��Ȼ�����Һ�а�ɫ�������� |
����˵������ȷ����
A. ���ɵĺ�ɫ�����к���CB. ��ɫ������Na2O
C. ʵ��˵��CO2����������D. ����Na�Ż�����CO2���
����Ŀ���������ʵ�����X��Y�������ij�ܱ������У���һ�������£��������·�Ӧ���ﵽƽ�⣺
X(g)+ 3Y(g) ![]() 2Z(g) ��H<0 ���ı�ij��������ά��������ֱ���µ�ƽ��ʱ���±��й�����ƽ����ԭƽ��ıȽ���ȷ����
2Z(g) ��H<0 ���ı�ij��������ά��������ֱ���µ�ƽ��ʱ���±��й�����ƽ����ԭƽ��ıȽ���ȷ����
ѡ�� | �ı����� | ��ƽ����ԭƽ��Ƚ� |
A | �����¶� | X��ת���ʱ�С |
B | ����ѹǿ | X��Ũ�ȱ�С |
C | ����һ����Y | Y��ת�������� |
D | ʹ���ʵ����� | X�����������С |
A. A B. B C. C D. D
����Ŀ����(Ti)����Ϊ��δ�����������㷺Ӧ���ڹ��������պ��졢������ϵ������ѵ��Ȼ���������ת���ϵ��2TiCl3![]() TiCl4��+TiCl2�ش��������⡣
TiCl4��+TiCl2�ش��������⡣
(1)ijͬѧ������̬ Cl-����Χ�����Ų�ͼΪ![]() ����Υ����____________
��������____________
(2)�ӽṹ�ǶȽ��� TiCl3��Ti(III)��ԭ�Խ�ǿ��ԭ��____________��
(3)�ѵ��Ȼ���IJ��������������±���
�Ȼ��� | �۵�/�� | �е�/�� | �ܽ��� |
TiCl4 | -24 | 136 | �����ڷǼ��Եļױ����ȴ��� |
TiCl2 | 1035 | 1500 | �������ȷ¡����� |
��TiCl4��TiCl2�ľ������ͷֱ���____________��
��TiCl4��SO42-��Ϊ�ȵ����壬��Ϊ����____________��ͬ��SO42-����ԭ����3s�����3p����ӻ���
(4)Ti��������ж��֡�Ti(CO)6��Ti(H2O)63+��TiF62-����������ԭ���е縺����С����__________��Ti(NO3)4������ṹ��ͼ��Ti����λ����_____________
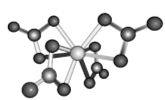
(5)���ѿ�(CaTiO3)����Ȼ���е�һ�ֳ�������侧���ṹ��ͼ��
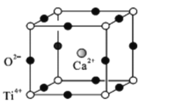
����NΪ�����ӵ�������ֵ������һ������������Ϊ______________g.
�ڼ���O2-���������������ܶѻ���Ti4+��O2-���У���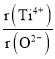 =_________��
=_________��