��Ŀ����
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�

��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
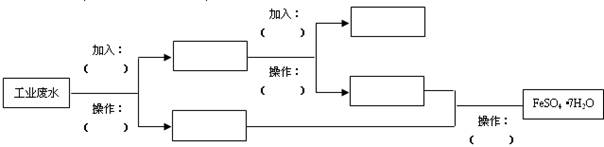
(1)���ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ_____________________________��
(2)�ڲ������б���pH��8��Ŀ����____________________________��
(3)���������Ҫ�ɷ���_______________________________________��
(4)�������м��ȡ���е�Ŀ����________________________________��
�˲������������������____________________________________��
(5)�����ܱ���pH��2��Ŀ����__________________________________��
�˲�����������õ���Ҫ������________________________________��

��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ_____________________________��
(2)�ڲ������б���pH��8��Ŀ����____________________________��
(3)���������Ҫ�ɷ���_______________________________________��
(4)�������м��ȡ���е�Ŀ����________________________________��
�˲������������������____________________________________��
(5)�����ܱ���pH��2��Ŀ����__________________________________��
�˲�����������õ���Ҫ������________________________________��
(1)Zn(NO3)2
4Zn��10HNO3===4Zn(NO3)2��N2O����5H2O
(2)��ֹ���ɵ�Zn(OH)2�������ܽ�
(3)Zn(OH)2��Fe(OH)3
(4)��ʹFe3����ȫˮ�⡡�¶�Խ�ߣ�ˮ��̶�Խ��
(5)����Zn2��ˮ��ΪZn(OH)2
�����ƾ��ơ�����̨��������
4Zn��10HNO3===4Zn(NO3)2��N2O����5H2O
(2)��ֹ���ɵ�Zn(OH)2�������ܽ�
(3)Zn(OH)2��Fe(OH)3
(4)��ʹFe3����ȫˮ�⡡�¶�Խ�ߣ�ˮ��̶�Խ��
(5)����Zn2��ˮ��ΪZn(OH)2
�����ƾ��ơ�����̨��������
��

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
�����Ŀ


 ��
��
 ɫ�� ɫ��
ɫ�� ɫ��
 �γ�һ��ˮ��
�γ�һ��ˮ��