��Ŀ����
13���������ʵ��������֪ʶ����д���пո���1�����飨CH4����Ħ������Ϊ16g/mol��8g CH4��Լ����0.5NA�����ӣ��ڱ�״������ռ�����ԼΪ11.2L��������ԭ������ȵļ���Ͱ�����NH3����������Ϊ12��17��ͬ�¡�ͬѹ�£�����Ͱ����ܶ�֮��Ϊ16��17��������ȵļ���Ͱ��������֮��Ϊ17��16��
��2����״���£�33.6L ���������CO��H2��������Ϊ49��4����CO�����Ϊ15.68L��
��3����30g�ܶ�Ϊdg/mL��AlCl3����Һ�к���0.9g Al3+��������Al3+��ˮ��Ӧ������Cl-Ũ��Ϊ$\frac{10d}{3}$mol/L
��4��ʵ���ҳ��õ�Ũ�����ܶ�Ϊ1.17g•mL-1����������Ϊ36.5%����Ũ��������ʵ���Ũ��Ϊ11.7mol/L������ȷ��С�����һλ��
���� ��1��Ħ��������g/molΪ��λ����ֵ�ϵ�������Է�������������n=$\frac{m}{M}$����������ʵ������ٸ���N=nNA������������Ŀ������V=nVm����������������Hԭ����Ŀ��ȼ�����顢�������ʵ���֮�ȣ�����m=nM���㰱�����������������������֮�ȣ�ͬ��ͬѹ�£�������ܶ�֮�ȵ�����Ħ������֮�ȣ�����n=$\frac{m}{M}$������顢���������ʵ���֮�ȣ�ͬ��ͬѹ�£��������֮�ȵ��������ʵ���֮�ȣ�
��2������n=$\frac{m}{M}$����CO��H2�����ʵ���֮�ȣ�����n=$\frac{V}{{V}_{m}}$����CO��H2�������ʵ�������������CO���ʵ������ٸ���V=nVm����CO�������
��3������V=$\frac{m}{��}$������Һ���������n=$\frac{m}{M}$����Al3+�����ʵ�������Һ��n��Cl-��=3n��Al3+�����ٸ���c=$\frac{n}{V}$����c��Cl-����
��4������c=$\frac{1000�Ѧ�}{M}$���㣮
��� �⣺��1�������Ħ������Ϊ16g/mol��8g CH4 �����ʵ���Ϊ$\frac{8g}{16g/mol}$=0.5mol�����з�����ĿΪ0.5mol��NAmol-1=0.5NA���ڱ�״������ռ�����ԼΪ0.5mol��22.4L/mol=11.2L��������ԭ������ȵļ���Ͱ�����NH3�������ʵ���֮��Ϊ$\frac{1}{4}$��$\frac{1}{3}$=3��4������������Ϊ3mol��16g/mol��4mol��17g/mol=12��17��ͬ��ͬѹ�£�������ܶ�֮�ȵ�����Ħ������֮�ȣ�����Ͱ����ܶ�֮��Ϊ16g/mol��17g/mol=16��17������n=$\frac{m}{M}$��֪��������ȵļ���Ͱ��������ʵ���֮��Ϊ17g/mol��16g/mol=17��16���������֮��Ϊ17��16��
�ʴ�Ϊ��16g/mol��0.5NA��11.2��12��17��16��17��17��16��
��2��CO��H2�����ʵ���֮��Ϊ$\frac{49}{28}$��$\frac{4}{2}$=49��56��CO��H2�������ʵ���Ϊ$\frac{33.6L}{22.4L/mol}$=1.5mol����CO���ʵ���Ϊ1.5mol��$\frac{49}{49+56}$����CO�����Ϊ1.5mol��$\frac{49}{49+56}$��22.4L/mol=15.68L��
�ʴ�Ϊ��15.68L��
��3����Һ���Ϊ$\frac{30g}{1000dg/L}$=$\frac{3}{100d}$L��Al3+�����ʵ���Ϊ$\frac{0.9g}{27g/mol}$=$\frac{1}{30}$mol����Һ��n��Cl-��=3n��Al3+��=0.1mol����c��Cl-��=$\frac{0.1mol}{\frac{3}{100d}L}$=$\frac{10d}{3}$mol/L��
�ʴ�Ϊ��$\frac{10d}{3}$mol/L��
��4������c=$\frac{1000�Ѧ�}{M}$��֪���ܶ�Ϊ1.17g•mL-1����������Ϊ36.5%��Ũ��������ʵ���Ũ��Ϊ$\frac{1000��1.17��36.5%}{36.5}$mol/L=11.7mol/L��
�ʴ�Ϊ��11.7mol/L��
���� ���⿼�����ʵ����йؼ��㣬ע�����������ʵ���Ϊ���ĵĻ����������ڻ���֪ʶ�Ĺ��̣�

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�| A�� | ����ͭ��Һ������������Һ��Ӧ��Ba2++SO42-�TBaSO4�� | |
| B�� | ����ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ����ˮ�ķ�Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| D�� | ��KHSO4��Һ�м���Ba��OH��2��Һ��ǡ�ó�����ȫ��Ba2++H++SO42-+OH-�TBaSO4��+H2O |
| A�� | �þƾ��Ƹ���ƿ������Ũ����Һ | |
| B�� | ����ʵ�鿪ʼʱ��ͨ������ˮ���ٵ�ȼ�ƾ��Ƽ��� | |
| C�� | ֻ�з�Һ©�����ܽ��е�ˮ�еĵ����ȡʵ�� | |
| D�� | Ũ��������Ƥ��Ӧ�ô���ˮ��ϴ��Ȼ��Ϳ����������������Һ |
 ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ�����Թܢ��м���ʯ���ͺ�����������ʯ���ͷֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м���������Ȼ�̼��Һ��
ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ�����Թܢ��м���ʯ���ͺ�����������ʯ���ͷֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м���������Ȼ�̼��Һ�� ��
�� ��
�� ���÷�Ӧ�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
���÷�Ӧ�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
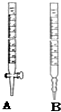 ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ��
ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ��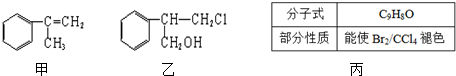

 +HCl$\stackrel{һ������}{��}$
+HCl$\stackrel{һ������}{��}$ ������ע����Ӧ��������
������ע����Ӧ��������
 ��
�� ��
�� ��
��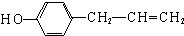 ��
��