��Ŀ����
��ҵ���ð������������������ᣬ���������̿ɱ�ʾΪ��
4NH3+5O2 4NO+6H2O��4NO+3O2+2H2O=4HNO3��
4NO+6H2O��4NO+3O2+2H2O=4HNO3��
��1������1.7��Һ��Ϊԭ����������������Ϊ50%�����ᣬ������������Ҫ��ˮ������Ϊ______�֣����������������з�Ӧ��������������ģ�
��2����ҵ����Mg��NO3��2���ŨH2SO4����Ϊ��ȡŨHNO3����ˮ��������50%��������������ͬ��������M1�֣������м���80%��Mg��NO3��2��ҺM2�֣��������õ�90%�������60%��Mg��NO3��2��Һ������HNO3���������������HNO3��Mg��NO3��2��H2O������ģ�������ǰ��Ͷ�ϱ�M1/M2=______��
��3�����Ṥҵ�����е�β�����ô�����Һ���գ��йصĻ�ѧ��ӦΪ��2NO2+Na2CO3=NaNO2+NaNO3+CO2�ۣ�NO+NO2+Na2CO3=2NaNO2+CO2�ܣ����������Ĵ�����Һ���������Ṥҵβ����NO��NO2����ÿ����22.4L����״���£�CO2ʱ������Һ��������44g�������Ṥҵβ����NO��NO2�����ʵ���֮�ȣ�
�⣺��1����������ѧ����ʽ��ӣ��ã�4NH3+8O2=4HNO3+4H2O����ȥ��һ����Լ��4��Ϊ��
NH3 +2O2 =HNO3 +H2O
17 63 18
1.7t X Y
X=6.3t Y=1.8t
��������ҪΪ50%����������Һ������Ϊ6.3��/50%=12.6�֣�����Ҫ�ӵ�ˮ������Ϊ��12.6-6.3-1.8=4.5�֣��ʴ�Ϊ��4.5��
��2���豻ת�Ƶ�ˮ������ΪX��
50%�����Ậˮ��Ϊ50%��80%������þ��Һ��ˮ��Ϊ20%��90%�����Ậˮ��Ϊ10%��60%������þ��Һ��ˮ��Ϊ40%����
������ˮ��0.5M1-X=��M1-X����0.1
��ã�M1= X
X
����þ��ˮ��0.2M2+X=��M2+X����0.4
��ã�M2=3X
�� =
= =
=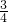 =0.75���ʴ�Ϊ��0.75��
=0.75���ʴ�Ϊ��0.75��
��3����NO�����ʵ�����x��NO2�����ʵ�����y����
x+ =
= mol=1mol��
mol=1mol��
32x+24��y-x��=44��
��ã�x=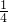 mol��y=
mol��y= mol����һ�������Ͷ������������ʵ���֮��
mol����һ�������Ͷ������������ʵ���֮�� =1��7��������ͬ�����£�һ�������Ͷ������������֮����1��7���ʴ�Ϊ��1��7��
=1��7��������ͬ�����£�һ�������Ͷ������������֮����1��7���ʴ�Ϊ��1��7��
��������1�������ܷ�Ӧ����ʽ��4NH3+8O2=4HNO3+4H2O����NH3+2O2=HNO3+H2O�����㣻
��2��������������б������ˮ���������ֱ��ҳ�����������þ��ת�Ƶ�ˮ�ĵ�����ϵ���ֱ���� M1��M2�����з�������⣻
��3�����ݶ�����̼�����������Һ�������ӵ����з�������һ�������Ͷ���������������Ӷ��ó������֮�ȣ�
������������Ҫ�����˻�ѧ���㣬ע��ƽʱ֪ʶ�Ļ����Լ����Ӧ���ǽ���Ĺؼ����Ѷ��еȣ�
NH3 +2O2 =HNO3 +H2O
17 63 18
1.7t X Y
X=6.3t Y=1.8t
��������ҪΪ50%����������Һ������Ϊ6.3��/50%=12.6�֣�����Ҫ�ӵ�ˮ������Ϊ��12.6-6.3-1.8=4.5�֣��ʴ�Ϊ��4.5��
��2���豻ת�Ƶ�ˮ������ΪX��
50%�����Ậˮ��Ϊ50%��80%������þ��Һ��ˮ��Ϊ20%��90%�����Ậˮ��Ϊ10%��60%������þ��Һ��ˮ��Ϊ40%����
������ˮ��0.5M1-X=��M1-X����0.1
��ã�M1=
 X
X����þ��ˮ��0.2M2+X=��M2+X����0.4
��ã�M2=3X
��
 =
= =
=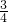 =0.75���ʴ�Ϊ��0.75��
=0.75���ʴ�Ϊ��0.75����3����NO�����ʵ�����x��NO2�����ʵ�����y����
x+
 =
= mol=1mol��
mol=1mol��32x+24��y-x��=44��
��ã�x=
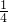 mol��y=
mol��y= mol����һ�������Ͷ������������ʵ���֮��
mol����һ�������Ͷ������������ʵ���֮�� =1��7��������ͬ�����£�һ�������Ͷ������������֮����1��7���ʴ�Ϊ��1��7��
=1��7��������ͬ�����£�һ�������Ͷ������������֮����1��7���ʴ�Ϊ��1��7����������1�������ܷ�Ӧ����ʽ��4NH3+8O2=4HNO3+4H2O����NH3+2O2=HNO3+H2O�����㣻
��2��������������б������ˮ���������ֱ��ҳ�����������þ��ת�Ƶ�ˮ�ĵ�����ϵ���ֱ���� M1��M2�����з�������⣻
��3�����ݶ�����̼�����������Һ�������ӵ����з�������һ�������Ͷ���������������Ӷ��ó������֮�ȣ�
������������Ҫ�����˻�ѧ���㣬ע��ƽʱ֪ʶ�Ļ����Լ����Ӧ���ǽ���Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 4NO+6H2O�� 4NO+3O2+2H2O��4HNO3��
4NO+6H2O�� 4NO+3O2+2H2O��4HNO3��