��Ŀ����
(1)�������Ȼ�̼���Ҵ��������dz������л��ܼ�������ˮ���ܵ���_____________������ˮ�����ܶȱ�ˮС����_________________________________��(2)һƿ��ɫ���壬���ܺ���CH4��CH2=CH2�����е�һ�֣���һƿCl2��Ϻ���գ��۲쵽����ɫ����ȥ��ƿ����������ɫ��״СҺ�Ρ�
��������ʵ�������ƶϳ���ƿ������һ������CH4������Ϊ�Ƿ���ȷ��Ϊʲô��
______________________________________��
������ʵ������漰�ķ�Ӧ������__________________________��
(3)����![]() �Ļ�������CH2=CH2һ������һ�������¿ɾۺϳɸ߷��ӻ����
�Ļ�������CH2=CH2һ������һ�������¿ɾۺϳɸ߷��ӻ����
�ٹ㷺����ũ�ñ�Ĥ�ľ�����ϩ���ϣ�����![]() �ۺ϶��ɵģ��仯ѧ����ʽ��____________________________________________��
�ۺ϶��ɵģ��仯ѧ����ʽ��____________________________________________��
�ڵ�����װ�д���ʹ�õ���ĭ���ϵ���Ҫ�ɷ��Ǿ۱���ϩ(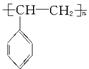 )��������_____________(д�ṹ��ʽ)�ۺ϶��ɵġ�
)��������_____________(д�ṹ��ʽ)�ۺ϶��ɵġ�
(1)�Ҵ� ��������
(2)�ٲ���ȷ����ΪCH2=CH2Ҳ������Cl2�����ӳɷ�Ӧ����ʹ����ɫ����ȥ�����ɵ�CH2ClCH2ClҲ����״Һ�塣��ȡ����Ӧ���ӳɷ�Ӧ


��ϰ��ϵ�д�
�����Ŀ
����˵��������ǣ�������
| A������ˮ���𱽡����Ȼ�̼���Ҵ�������ɫҺ�� | B��ȡ1 gNaOH�������ձ�������9 mLˮ����=1g?cm-3����ֽ������10%��NaOH��Һ | C����NaOH��Һ�Ϳɼ���K+��Mg2+��Al3+��Fe2+��Fe3+�������� | D��Ϊȷ�ⶨ������NaOH��Һ��Ӧ���к��ȣ�������ͼ�����ʵ���Ӧ��� |




