��Ŀ����
��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��| ʵ����� | ʱ�䣨min�� | ������ƽ�Ķ�����g�� |
| �ձ�+NaOH��Һ | 120.0 | |
| �ձ�+NaOH��Һ+��Ʒ | 135.6 | |
| 1 | 135.1 | |
| 2 | 134.7 | |
| 3 | 134.4 | |
| 4 | 134.4 |
��2����Ӧ����Һ�е�c��OH-�������ٶ���Ӧǰ����Һ������䣩
��3�������壺ƽ����Ӧ���ʨTij���ʷ����仯�����ʵ���/��Ӧ������ʱ�䣬����H2���ʵ����ı仯����ʾ��������Ӧ�ӿ�ʼ������ʱ��ƽ������Ϊ���٣�

���𰸡���������1���ȸ��ݵ�����ƽ�Ķ����ó���Ʒ������Ϊ15.6g������NaOH��Һ��Ӧ����þ��NaOH��Һ����Ӧ���ձ�+NaOH��Һ+��Ʒ�������ٵ�����������������NaOH��Һ��Ӧ���������������������þ������������
��2������NaOH��Һ��Ӧ����þ��NaOH��Һ����Ӧ���ձ�+NaOH��Һ+��Ʒ�������ٵ�����������������NaOH��Һ��Ӧ�����������NaOH�����ʵ�������������Һ�е�c��OH-����
��3������ƽ������V=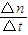 ��������
��������
����⣺��1����Ʒ������Ϊ15.6g��NaOH�����ʵ���Ϊ��4.5mol/L×0.1L=0.45mol������NaOH��Һ��Ӧ����������������Ϊ135.6g-134.4g=1.2g�������ʵ���Ϊ0.6mol��
2Al+2NaOH+2H2O=2NaAlO2 +3H2��
2 2 3
0.4mol 0.4mol 0.6mol
NaOH��Һ��������ȫ���μӷ�Ӧ����������Ϊ 0.4mol×27g/mol=10.8g����þ������Ϊ15.6g-10.8g=4.8g������þ����������Ϊ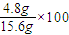 %=30.8%��
%=30.8%��
�𣺼�����Ʒ��þ����������30.8%��
��2����Ʒ������Ϊ15.6g��NaOH�����ʵ���Ϊ��4.5mol/L×0.1L=0.45mol������NaOH��Һ��Ӧ����������������Ϊ135.6g-134.4g=1.2g�������ʵ���Ϊ0.6mol��
2Al+2NaOH+2H2O=2NaAlO2 +3H2��
2 2 3
0.4mol 0.4mol 0.6mol
NaOH��Һ��������ȫ���μӷ�Ӧ��������NaOHΪ0.45mol-0.4mol=0.05mol����Ӧ����Һ�е�c��OH-��=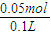 =0.5mol/L���ʴ�Ϊ����Ӧ����Һ�е�c��OH-��Ϊ0.5mol/L��
=0.5mol/L���ʴ�Ϊ����Ӧ����Һ�е�c��OH-��Ϊ0.5mol/L��
��3��ƽ������V=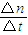 =
=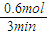 =0.2mol/min��
=0.2mol/min��
����H2���ʵ����ı仯����ʾ��������Ӧ�ӿ�ʼ������ʱ��ƽ������Ϊ0.2mol/min��
������������Ҫ���������ʳɷֺ����ļ��㣬ץס����NaOH��Һ��Ӧ����þ��NaOH��Һ����Ӧ�ǽ���Ĺؼ����Ѷ��еȣ�
��2������NaOH��Һ��Ӧ����þ��NaOH��Һ����Ӧ���ձ�+NaOH��Һ+��Ʒ�������ٵ�����������������NaOH��Һ��Ӧ�����������NaOH�����ʵ�������������Һ�е�c��OH-����
��3������ƽ������V=
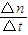 ��������
������������⣺��1����Ʒ������Ϊ15.6g��NaOH�����ʵ���Ϊ��4.5mol/L×0.1L=0.45mol������NaOH��Һ��Ӧ����������������Ϊ135.6g-134.4g=1.2g�������ʵ���Ϊ0.6mol��
2Al+2NaOH+2H2O=2NaAlO2 +3H2��
2 2 3
0.4mol 0.4mol 0.6mol
NaOH��Һ��������ȫ���μӷ�Ӧ����������Ϊ 0.4mol×27g/mol=10.8g����þ������Ϊ15.6g-10.8g=4.8g������þ����������Ϊ
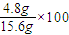 %=30.8%��
%=30.8%���𣺼�����Ʒ��þ����������30.8%��
��2����Ʒ������Ϊ15.6g��NaOH�����ʵ���Ϊ��4.5mol/L×0.1L=0.45mol������NaOH��Һ��Ӧ����������������Ϊ135.6g-134.4g=1.2g�������ʵ���Ϊ0.6mol��
2Al+2NaOH+2H2O=2NaAlO2 +3H2��
2 2 3
0.4mol 0.4mol 0.6mol
NaOH��Һ��������ȫ���μӷ�Ӧ��������NaOHΪ0.45mol-0.4mol=0.05mol����Ӧ����Һ�е�c��OH-��=
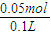 =0.5mol/L���ʴ�Ϊ����Ӧ����Һ�е�c��OH-��Ϊ0.5mol/L��
=0.5mol/L���ʴ�Ϊ����Ӧ����Һ�е�c��OH-��Ϊ0.5mol/L����3��ƽ������V=
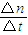 =
=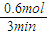 =0.2mol/min��
=0.2mol/min������H2���ʵ����ı仯����ʾ��������Ӧ�ӿ�ʼ������ʱ��ƽ������Ϊ0.2mol/min��
������������Ҫ���������ʳɷֺ����ļ��㣬ץס����NaOH��Һ��Ӧ����þ��NaOH��Һ����Ӧ�ǽ���Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��
��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��
| ʵ����� | ʱ�䣨min�� | ������ƽ�Ķ�����g�� |
| �ձ�+NaOH��Һ | 120.0 | |
| �ձ�+NaOH��Һ+��Ʒ | 0 | 135.6 |
| 1 | 135.1 | |
| 2 | 134.7 | |
| 3 | 134.4 | |
| 4 | 134.4 |
��2����Ӧ����Һ�е�c��OH-�������ٶ���Ӧǰ����Һ������䣩
��3�������壺ƽ����Ӧ���ʨTij���ʷ����仯�����ʵ���/��Ӧ������ʱ�䣬����H2���ʵ����ı仯����ʾ��������Ӧ�ӿ�ʼ������ʱ��ƽ������Ϊ���٣�
 ������������������Һ��
������������������Һ�� ��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��
��ͼ��ʾ�Dzⶨþ�ۣ�������������Al�����ȵ�ʵ��װ�ã�����NaOH��Һ��Ũ��Ϊ4.5mol/L�����Ϊ100mL����ͬʱ��ε�����ƽ�Ķ������±���ʾ��