��Ŀ����
����ͼ1װ�ÿ��Խ��вⶨSO2ת����SO3��ת���ʵ�ʵ�飮����ͼ1װ�ý���SO2ת��ΪSO3��ת���ʲⶨʵ�飺2SO2+O2| Cr2O3 |
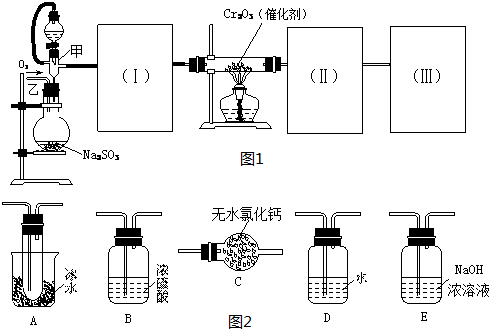
��1��Ҫ˳������ʵ�飬ͼ1�Т�Ӧ����1�����ʵ�װ�ã����ͼ2��ѡ�����˵�װ�ã������������ո��ڣ�
��
��2��ʵ��ǰ��������еIJ����ǣ���������ƣ�����д������̣�
��3��ʵ��ʱ��Ũ������˳��������ƿ�У���װ���������õ�ԭ���ǣ�
��4�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳����
��5��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ����ô�������Cr2O3���ķ�Ӧ��ʱ��SO2��ת���ʻ�
��6��ʵ��ʱ����25.2g��Na2SO3�������Ũ�����������ģ���Ӧ����ʱ����ͨ��O2һ��ʱ�䣬�Ƶâ�װ�õ���������13.68g����ʵ����SO2��ת����Ϊ
��������1����������ĸ�����Ũ���ᣬ���¶ȵ���16.8����Ի����������ľ��壬β���������ռ���Һ��
��2��������Ʊ�������ʵ������Ҫ���װ�õ������ԣ�
��3������ѹǿʹҺ��˳�����£�
��4��Ϊ��֤���ɵĶ��������ܵ�ת��Ϊ��������Ӧ�ȼ��ȴ����ٽ��еμ�Ũ���������
��5����ӦΪ���ȷ�Ӧ��
��6������Na2SO3��SO2��SO3��ϵʽ�����м��㣮
��2��������Ʊ�������ʵ������Ҫ���װ�õ������ԣ�
��3������ѹǿʹҺ��˳�����£�
��4��Ϊ��֤���ɵĶ��������ܵ�ת��Ϊ��������Ӧ�ȼ��ȴ����ٽ��еμ�Ũ���������
��5����ӦΪ���ȷ�Ӧ��
��6������Na2SO3��SO2��SO3��ϵʽ�����м��㣮
����⣺��1��SO2ת����SO3֮ǰ����Զ���������и��������Ũ���ᣬSO3���۵���16.8�棬�е���44.8�棬���¶ȵ��� 16.8��ʱ�����������Ծ���״̬���ڣ������������������β���������ռ���Һ���գ�
�ʴ�Ϊ��B��A��E��
��2��ʵ��ǰ��������еIJ����Ǽ��װ�õ������ԣ��ʴ�Ϊ�����װ�õ������ԣ�
��3��ΪʹҺ��˳�����£����װ������������DZ��ַ�Һ©����ѹǿ����ƿ��ѹǿ��ȣ�
�ʴ�Ϊ�����ַ�Һ©����ѹǿ����ƿ��ѹǿ��ȣ�
��4��Ϊ��֤���ɵĶ��������ܶ��ת��Ϊ��������Ӧ�ȼ��ȴ����ٽ��еμ�Ũ�����������֤���ɵĶ��������ܵIJ��뷴Ӧ��
�ʴ�Ϊ���ȼ��ȴ�����Ȼ�����μ�Ũ���
��5����ӦΪ���ȷ�Ӧ���¶ȹ��߲����ö�����������ɣ����Ͷ��������ת���ʣ�
�ʴ�Ϊ�����ͣ�
��6������ԭ���غ��֪��Na2SO3��SO2��SO3��n��Na2SO3��=
=0.2mol��������n��SO3��=0.2mol��������Ϊ0.2mol��80g/mol=16g���Ƶâ�װ�õ���������13.68g����SO2��ת����Ϊ
��100%=85.5%��
�ʴ�Ϊ��85.5%��
�ʴ�Ϊ��B��A��E��
��2��ʵ��ǰ��������еIJ����Ǽ��װ�õ������ԣ��ʴ�Ϊ�����װ�õ������ԣ�
��3��ΪʹҺ��˳�����£����װ������������DZ��ַ�Һ©����ѹǿ����ƿ��ѹǿ��ȣ�
�ʴ�Ϊ�����ַ�Һ©����ѹǿ����ƿ��ѹǿ��ȣ�
��4��Ϊ��֤���ɵĶ��������ܶ��ת��Ϊ��������Ӧ�ȼ��ȴ����ٽ��еμ�Ũ�����������֤���ɵĶ��������ܵIJ��뷴Ӧ��
�ʴ�Ϊ���ȼ��ȴ�����Ȼ�����μ�Ũ���
��5����ӦΪ���ȷ�Ӧ���¶ȹ��߲����ö�����������ɣ����Ͷ��������ת���ʣ�
�ʴ�Ϊ�����ͣ�
��6������ԭ���غ��֪��Na2SO3��SO2��SO3��n��Na2SO3��=
| 25.2 |
| 126 |
| 13.68 |
| 16 |
�ʴ�Ϊ��85.5%��
���������⿼�������������ʡ�����ȵ�֪ʶ���ۺ��Խ�ǿ����Ŀ�ѶȽϴ��ص㿼��ѧ�������ͽ�������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

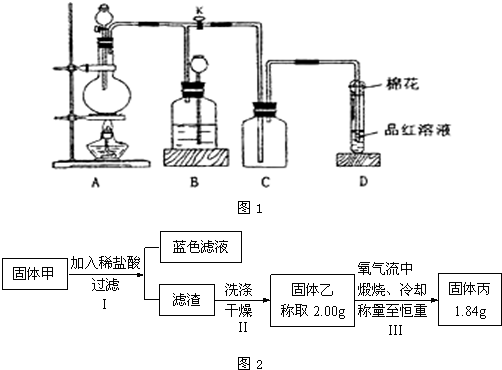
 ��ҵ�����Ĵ����г���������NaCl���ʣ�Ϊ�ⶨij������Ʒ�Ĵ��ȣ���ѧ����С�����������ʵ�鷽����
��ҵ�����Ĵ����г���������NaCl���ʣ�Ϊ�ⶨij������Ʒ�Ĵ��ȣ���ѧ����С�����������ʵ�鷽����