��Ŀ����
 ����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ��������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֣�ij��ѧ����С��ͨ�����һ̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ���������ͼ��ʾ��
����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ��������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֣�ij��ѧ����С��ͨ�����һ̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ���������ͼ��ʾ��
[�������]
����������ijɷֿ���ֻ��______һ�֣�
����������ijɷֿ��ܺ���______���֣�
����������ijɷֿ��ܺ���SO2��SO3��O2���֣�
[ʵ��̽��]ʵ��������̣��ԣ�����֪ʵ�����ʱ������ͭ��ȫ�ֽ⣮
��1��������װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ
______ ������š��������ظ�ʹ�ã���
��2��ʵ������У�����C��������______��
[��֤���裬��������]
��3��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
| ʵ�� С�� | ��ȡCuSO4 ������/g | ����C�� �ӵ�����/g | ��Ͳ��ˮ���������� ״������������/mL |
| �� | 6.4 | 2.56 | 224 |
| �� | 6.4 | 2.56 | 448 |
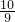 �����������������ƽ����Է�������Ϊ______������һλС������ָ����ʦ��Ϊ��������װ�ò�õ�����C������ƫС���������ɿ�����______��
�����������������ƽ����Է�������Ϊ______������һλС������ָ����ʦ��Ϊ��������װ�ò�õ�����C������ƫС���������ɿ�����______��
 2CuO+2SO2��+O2���� CuSO4
2CuO+2SO2��+O2���� CuSO4 CuO+SO3������������Ӧͬʱ�������жϣ��ʴ�Ϊ����SO3 ��SO2��O2
CuO+SO3������������Ӧͬʱ�������жϣ��ʴ�Ϊ����SO3 ��SO2��O2��1������Ʊ����ռ����շ���װ�á���������װ�á���ˮ������װ������װʵ���������ʴ�Ϊ���٢ۢܢ�ߢڣ�
��2��SO2��SO3��������������ʷ�Ӧ���ü�ʯ�ҿ�����SO2��SO3���壬�ʴ�Ϊ������SO2��SO3���壻
��3������6.4g����ͭ�ֽ�����xmolSO3��ymolSO2������
80x+64y=2.56
x+y=
 =0.04
=0.04��֮�ã�x=0��y=0.04mol����n��O2��=
 =0.02mol�����Զ��ߵ����ʵ���֮��Ϊ2��1������ʽ��2CuSO4
=0.02mol�����Զ��ߵ����ʵ���֮��Ϊ2��1������ʽ��2CuSO4
2CuO+2SO2��+O2�����ʴ�Ϊ��2CuSO4
 2CuO+2SO2��+O2����
2CuO+2SO2��+O2��������6.4g����ͭ�ֽ�����xmolSO3��ymolSO2������
80x+64y=2.56
x+y=
 =0.04
=0.04��֮�ã�x=0��y=0.04mol����n��O2��=
 =0.01mol��
=0.01mol��2SO2 +O2 ?2SO3
��ʼ��mol�� 0.04 0.01 0
��Ӧ��mol�� 2a a 2a
ƽ�⣨mol�� 0.04-2a 0.01-a 2a
����
 =
=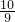 �����a=0.005�����������ƽ��Ħ������Ϊ=
�����a=0.005�����������ƽ��Ħ������Ϊ=
=64.0���ʴ�Ϊ��64.0��
��������δ��ȫ��Ӧ������ʣ�࣬�ʴ�Ϊ��Cװ��ǰ��װ���ڲ����˲���SO2���壮
������[�������]��������ͭ�ֽ���ܷ����������2CuSO4
 2CuO+2SO2��+O2���� CuSO4
2CuO+2SO2��+O2���� CuSO4 CuO+SO3������������Ӧͬʱ�������жϣ�
CuO+SO3������������Ӧͬʱ�������жϣ���1������������Ʊ����ռ����շ���װ�á���������װ�á���ˮ������װ������װʵ��������
��2��SO2��SO3��������������ʷ�Ӧ���ü�ʯ�ҿ�����SO2��SO3���壻
��3���ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ���ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��Ȼ��������һ�������·�Ӧ��2SO2+O2?2SO3���ָ�����Ӧǰ���¶Ⱥ�ѹǿʱ���ܶ�֮�ȵ������ʵ����ķ���������Ե����ʵ����������������ƽ��Ħ��������
��������δ��ȫ��Ӧ������ʣ�࣮
���������⿼�����ʵ���ɺ�ʵ�����ݵĴ���������ʱע�����ʵ�����֪ʶ����������й����ݽ��м��㣬�������һ���Ѷȣ�

������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��1����ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2��
�ٲ��ij��ͭ��(CuFeS2)�к���20%����������������ÿ�ʯ��ͭ������������
������һ����Ȼ��ͭ��������ʯ����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺��ȡ
��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ����н������գ�����Cu��Fe3O4��SO2���壬��100 mL���е��۵�
����ˮȫ������SO2��Ȼ��ȡ10mL����Һ����0.05mol/L������Һ���еζ�����ȥ������Һ����
��Ϊ20.00mL����û�ͭ��Ĵ��ȡ�
��2����FeS��Fe2O3�Ļ����56.6 g��������ϡH2SO4�ܽ��ɵ�3.2 g��ԭ�������FeS��������
��3��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����
������Һ���ա�������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⡣����ˮ����ͭ��ĩ����Ϊ10.0 g��
��ȫ�ֽ��װ�õ������仯��ϵ���±���ʾ��
|
װ�� |
A���Թ�+��ĩ�� |
B |
C |
|
��Ӧǰ |
42.0 g |
75.0 g |
140.0 g |
|
��Ӧ�� |
37.0 g |
79.0 g |
140.5 g |

��ͨ�����㣬�ƶϳ���ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ��
��4������������Ƥ�����Ҫ��ѧ�Լ���������ˮNa2SO4��̿���ڸ����·�Ӧ�Ƶã���ѧ����ʽ���£�
��Na2SO4
+ 4C Na2S + 4CO�� ��Na2SO4
+ 4CO
Na2S + 4CO�� ��Na2SO4
+ 4CO Na2S + 4CO2
Na2S + 4CO2
a.���ڷ�Ӧ�����У�����CO��CO2�������Ϊ2mol��������Na2S�����ʵ�����
b.���ƾ�������ڿ����У��Ỻ��������Na2SO3��������Na2SO4���ֽ�43.72g���ֱ��ʵ�������Ʒ����ˮ�У���������������˵�4.8g������1.12L H2S ���壨��״����������Һ������ȫ���ݳ���������Һ�м���������BaCl2����˵�2.33g������������������Ʒ�ijɷּ������ʵ�����
 ������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��1����ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2��
�ٲ��ij��ͭ��CuFeS2���к���20%����������������ÿ�ʯ��ͭ������������
������һ����Ȼ��ͭ��������ʯ����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ����н������գ�����Cu��Fe3O4��SO2���壬��100mL���е��۵�����ˮȫ������SO2��Ȼ��ȡ10mL����Һ����0.05mol/L������Һ���еζ�����ȥ������Һ�����Ϊ20.00mL����û�ͭ��Ĵ��ȣ�
��2����FeS��Fe2O3�Ļ����56.6g��������ϡH2SO4�ܽ��ɵ�3.2g����ԭ�������FeS��������
��3��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����������Һ���գ�������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⣮����ˮ����ͭ��ĩ����Ϊ10.0g����ȫ�ֽ��װ�õ������仯��ϵ���±���ʾ��
| װ�� | A ���Թ�+��ĩ�� | B | C |
| ��Ӧǰ | 42.0g | 75.0g | 140.0g |
| ��Ӧ�� | 37.0g | 79.0g | 140.5g |
��4������������Ƥ�����Ҫ��ѧ�Լ���������ˮNa2SO4��̿���ڸ����·�Ӧ�Ƶã���ѧ����ʽ���£�
��Na2SO4+4C
 Na2S+4CO�� ��Na2SO4+4CO
Na2S+4CO�� ��Na2SO4+4CO Na2S+4CO2
Na2S+4CO2�����ڷ�Ӧ�����У�����CO��CO2�������Ϊ2mol��������Na2S�����ʵ�����
�����ƾ�������ڿ����У��Ỻ��������Na2SO3��������Na2SO4���ֽ�43.72g���ֱ��ʵ�������Ʒ����ˮ�У���������������˵�4.8g������1.12L H2S ���壨��״����������Һ������ȫ���ݳ���������Һ�м���������BaCl2����˵�2.33g������������������Ʒ�ijɷּ������ʵ�����
��1����ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2��
�ٲ��ij��ͭ��CuFeS2���к���20%����������������ÿ�ʯ��ͭ������������
������һ����Ȼ��ͭ��������ʯ����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ����н������գ�����Cu��Fe3O4��SO2���壬��100mL���е��۵�����ˮȫ������SO2��Ȼ��ȡ10mL����Һ����0.05mol/L������Һ���еζ�����ȥ������Һ�����Ϊ20.00mL����û�ͭ��Ĵ��ȣ�
��2����FeS��Fe2O3�Ļ����56.6g��������ϡH2SO4�ܽ��ɵ�3.2g����ԭ�������FeS��������
��3��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����������Һ���գ�������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⣮����ˮ����ͭ��ĩ����Ϊ10.0g����ȫ�ֽ��װ�õ������仯��ϵ���±���ʾ��
| װ�� | A ���Թ�+��ĩ�� | B | C |
| ��Ӧǰ | 42.0g | 75.0g | 140.0g |
| ��Ӧ�� | 37.0g | 79.0g | 140.5g |
��4������������Ƥ�����Ҫ��ѧ�Լ���������ˮNa2SO4��̿���ڸ����·�Ӧ�Ƶã���ѧ����ʽ���£�
��Na2SO4+4C
 Na2S+4CO ��Na2SO4+4CO
Na2S+4CO ��Na2SO4+4CO Na2S+4CO2
Na2S+4CO2�����ڷ�Ӧ�����У�����CO��CO2�������Ϊ2mol��������Na2S�����ʵ�����
�����ƾ�������ڿ����У��Ỻ��������Na2SO3��������Na2SO4���ֽ�43.72g���ֱ��ʵ�������Ʒ����ˮ�У���������������˵�4.8g������1.12L H2S ���壨��״����������Һ������ȫ���ݳ���������Һ�м���������BaCl2����˵�2.33g������������������Ʒ�ijɷּ������ʵ�����

 ��2013?բ������ģ��������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��2013?բ������ģ��������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
 ��
�� ��Һ����ʱ������
��Һ����ʱ������ ����
����