��Ŀ����
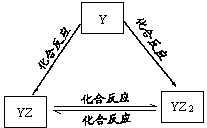
X��Y��Z��M��G����Ԫ�ط�������������,��ԭ��������������X��Zͬ����,���γ����ӻ�����ZX ; Y��Mͬ����,���γ�MY2��MY3���ַ��ӡ�
��1��Y��Ԫ�����ڱ��е�λ��Ϊ���������������� ��
��2������Ԫ�ص�����������Ӧ��ˮ����������ǿ������������ (д��ѧʽ),�ǽ�����̬�⻯�ﻹԭ����ǿ������������ (д��ѧʽ)��
��3��X��Y��Z��M��������ε���Һ��Ӧ�ɲ���MY2���壬д���䷴Ӧ���ӷ���ʽ������������������
��4��M����������G�ĵ��ʵ�ˮ��Һ����Ư���ԣ���ͬ�����£���ͬ�����M����������Y�ĵ��ʻ��ͨ��Ʒ����Һ��Ʒ����Һ������������ɫ����ɫ����ԭ���û�ѧ����ʽ��ʾ������������������
��1���ڶ�����VIA�� ��2��HClO4 H2S ��3��HSO3-+H+ = SO2��+ H2O
��4������ɫ �� Cl2+SO2 +2H2O =H2SO4 +2HCl
���������������1���������֪X��H��Y��O��Z��Na��M��S��G��Cl����Y��Ԫ�����ڱ��е�λ����λ�ڵڶ�����VIA�壬��2�����ڷǽ�������ǿ��Ԫ����Cl������������������Ӧ��ˮ����������ǿ����HClO4���ǽ�����̬�⻯�ﻹԭ����ǿ����H2S����3��MY2ΪSO2����������Ԫ����ɵ���������ΪNaHSO4��NaHSO3��NaHSO4��ˮ��Һ�пɵ����H+��һԪǿ������ã���Ӧ�����ӷ���ʽΪ��HSO3-+H+ = SO2��+ H2O����4�� )M����Ư���Ե�������ΪSO2��G�ĵ���ΪCl2����ΪSO2�л�ԭ�� ��Cl2�������ԡ�������1:1�����ʵ����ıȻ�ϣ���ˮ�з���ǡ��������ԭ��ӦCl2+ SO2+ 2H2O=2HCl+H2SO4������û��Ư���Ե�HCl��H2SO4�����ʧȥƯ��������
���㣺����Ԫ�ص��ƶϼ�SO2��Cl2��Ư���ԡ�H2S��ԭ�Ե����ʵ�֪ʶ��

���л�ѧʵ����ʵ������۶���ȷ���ǣ� ��
| ѡ�� | ʵ����ʵ | ���� |
| A | ��SO2ͨ�뺬HClO����Һ������H2SO4 | HClO�����Ա�H2SO4ǿ |
| B | �����ھƾ��ƻ����ϼ����ۻ��������� | ���������������۵������ |
| C | SiO2���Ժ�NaOH��Һ��HF��Һ��Ӧ | SiO2�������������� |
| D | ��SO2ͨ����ˮ�У���ˮ��ɫ | SO2����Ư���� |
��ҵ���������̿���Ҫ�ɷ�ΪMnO2��ͬʱ�������������ȵĻ�����Ʊ������̵ij����������£�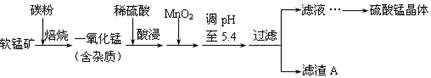
���ֽ���������������������ʽ��ȫ����ʱ��Һ��pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 |
��1��һ���������������ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ____________________����������MnO2����Һ�е�Fe2��������Fe3������Ŀ����___________��
��2������A�ijɷֳ�MnO2�⣬����_______________��
��3��MnO2���������п�̵�صĻ���ԭ�ϣ��ŵ�ʱ�����ĵ缫��ӦʽΪ________����ҵ����ʯīΪ�缫����ữ��MnSO4��Һ����MnO2�������ĵ缫��ӦʽΪ_________������������4.48L�����������ʱ��MnO2�����۲���Ϊ______g��
��4���̵��������ܻ�������ܶȻ���Ksp(MnCO3)��1.8��10��11��Ksp[Mn(OH)2]��1.9��10��13��Ksp(MnS)��2.0��10��13������������������ı�����Һ�У�Mn2��Ũ���ɴ�С��˳����_______��_______��_______����д��ѧʽ����
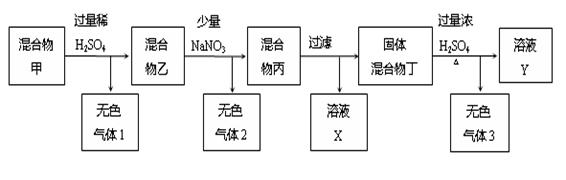
 ��X�е�һ�֡�
��X�е�һ�֡� C��CH3COO D��SiO
C��CH3COO D��SiO