��Ŀ����
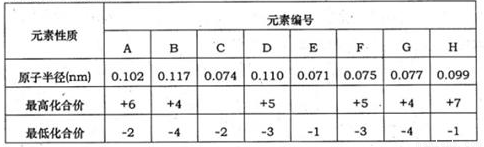
�±�Ϊ���ֶ�����Ԫ�����ʵ����ݣ�

(1)д������Ԫ�ص�Ԫ�ط��ţ�C________ ��D _______��I____��
(2)A��B��ԭ�Ӹ�����2��1���һ�ֻ�����ף�B��C��ԭ�Ӹ�����1��1���һ�ֻ������ң������ҷ�����Ӧ�Ļ�ѧ����ʽΪ____��
(3)E��G�ĵ��ʳ���������Ͻ�ijͬѧΪ�ⶨij��E��G�Ͻ���E���ʵĺ������ڱ�״���½��мס��ҡ�������ʵ�飺 �����ȡ30.0mLͬŨ�ȵ����ᣬ����úϽ���Ʒ��ĩ���������壬�й��������±���ʾ����úϽ���E���ʵ���������Ϊ__________________ ��
(2)A��B��ԭ�Ӹ�����2��1���һ�ֻ�����ף�B��C��ԭ�Ӹ�����1��1���һ�ֻ������ң������ҷ�����Ӧ�Ļ�ѧ����ʽΪ____��
(3)E��G�ĵ��ʳ���������Ͻ�ijͬѧΪ�ⶨij��E��G�Ͻ���E���ʵĺ������ڱ�״���½��мס��ҡ�������ʵ�飺 �����ȡ30.0mLͬŨ�ȵ����ᣬ����úϽ���Ʒ��ĩ���������壬�й��������±���ʾ����úϽ���E���ʵ���������Ϊ__________________ ��

(1)Na��S��P
(2)2H2O+2Na2O2==4NaOH+O2��
(3)47.1%
(2)2H2O+2Na2O2==4NaOH+O2��
(3)47.1%

��ϰ��ϵ�д�
�����Ŀ


 �±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺