��Ŀ����
��ͼ��A��B��C��D��E��F�ȼ��ֳ����л���֮���ת����ϵͼ������A����۵���Ҫ�ɷ֣�C��E��Ӧ������F��F������ζ��B��E��ʵ��ʽ��ͬ
![]()
���л����У����Ǿ���-CHO�ṹ�����ʣ������������ʣ�
��1�������Ƶ�������ͭ����Һ��Ӧ������ש��ɫ�ij�����
��2���ڴ����������£�-CHO����������Ϊ-COOH,����
2R-CHO+O2![]() 2R-COOH
2R-COOH
���ݸ���������Ϣ�������ʵ�ת����ϵ������и��⣺
(1) A�Ļ�ѧʽΪ ��B�Ľṹ��ʽΪ���������� ����������B����ͬ���칹���һ�����ʵ����� ��
(2) F��ϡ�����з���ˮ�ⷴӦ�ķ�Ӧ����ʽ
(3) E�백ˮ���������ӷ���ʽ
E��С�մ���Һ��Ӧ�Ļ�ѧ����ʽΪ�������������������������� ����
(4) ������������������ͭ����Һ����ש��ɫ�ij����������� �������ƣ���
(5) д������C�ķ�Ӧ����ʽ
(6) д��C��D�ķ�Ӧ����ʽ
(7) C+E��F�ķ�Ӧ����ʽ
���𰸡���1����C6H10O5���� CH2OH��CHOH��4CHO ����
��2��CH3COOC2H5+H2O![]() CH3COOH+C2H5OH
CH3COOH+C2H5OH
��3��CH3COOH+NH3.H2O��CH3COO-+NH4++H2O
CH3COOH+NaHCO3��CH3COONa+CO2��+H2O
��4��BE
(5)2CH3CH2OH+2Na��2CH3CH2ONa+H2��
(6) 2CH3CH2OH+O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O
(7) C2H5OH+CH3COOH![]() CH3COOC2H5+H2O
CH3COOC2H5+H2O
�����������ƶ����ͻ�ƿ��ڡ���ۡ��͡�B��E��ʵ��ʽ��ͬ������۵���Ҫ�ɷ��ǵ��ۡ�ͨ����Ŀ��ʾ����֪AΪ���ۣ�����A���ˮ�⣬����ˮ�����Ϊ���ǣ���BΪ�����ǣ�ͨ��C����D��������֪CΪ����DΪȩ����ϾƵ����ƣ���֪C��D�ֱ�Ϊ�Ҵ�����ȩ����EΪ�������ΪC��E����F����FΪ������������F��ϡ�����е�ˮ�ⷴӦ��������������ˮ�⡣Ȼ�������ĿҪ�������𰸡�

 С�����ϵ�д�
С�����ϵ�д�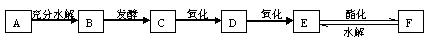
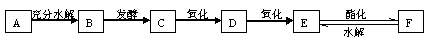
 ��ͼ��A��B��C��D��E��F�������ʵ�ת����ϵ������B��һ�ֳ�������ɫ��ζ��Һ�壬C��һ���д��ԵĻ����E��һ����ɫ��ζ���ж����塣
��ͼ��A��B��C��D��E��F�������ʵ�ת����ϵ������B��һ�ֳ�������ɫ��ζ��Һ�壬C��һ���д��ԵĻ����E��һ����ɫ��ζ���ж����塣