��Ŀ����
ϡ���������������Ӧ�Ļ�ѧ����ʽ���£�3Fe+8HNO3=3Fe��NO3��2+2NO��+4H2O
��1����������ԭ��Ӧ����������______������������______����˫���ŷ��ڻ�ѧ����ʽ�б�ʾ������ת�Ƶķ������ĿΪ��3Fe+8HNO3=3Fe��NO3��2+2NO��+4H2O
��2���˷�Ӧ�����ӷ���Ϊ______��
��3�������ɱ�״����11.2LNO���壬����μӷ�Ӧ�����������Լ���Ӧ�б���ԭ��HNO3�����ʵ�����
��1����������ԭ��Ӧ����������______������������______����˫���ŷ��ڻ�ѧ����ʽ�б�ʾ������ת�Ƶķ������ĿΪ��3Fe+8HNO3=3Fe��NO3��2+2NO��+4H2O
��2���˷�Ӧ�����ӷ���Ϊ______��
��3�������ɱ�״����11.2LNO���壬����μӷ�Ӧ�����������Լ���Ӧ�б���ԭ��HNO3�����ʵ�����
��1���÷�Ӧ�У�����õ���������������ʧ��������ԭ��������Ӧ�IJ���������������������÷�Ӧ����ʧȥ������=3��2-0��=6������õ�����=2��5-2��=6��������˫���ŷ��ڻ�ѧ����ʽ�б�ʾ������ת�Ƶķ������ĿΪ
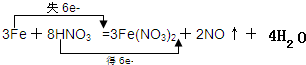
��
�ʴ�Ϊ��HNO3��Fe��NO3��2��
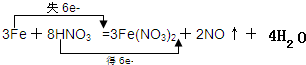
��
��2���÷�Ӧ�����ӷ���ʽΪ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O���ʴ�Ϊ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
��3����μӷ�Ӧ����������Ϊm��
����ԭ��HNO3�����ʵ���Ϊn
�� 3Fe+8HNO3=3Fe��NO3��2+2NO��+4H2O
3��56g 2mol������ԭ�� 2��22.4L
m n 11.2L
�� m=
=42 g
n=
=0.5 mol
�𣺲μӷ�Ӧ����������42g������ԭ����������ʵ�����0.5mol��
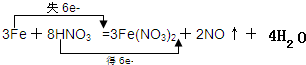
��
�ʴ�Ϊ��HNO3��Fe��NO3��2��
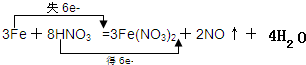
��
��2���÷�Ӧ�����ӷ���ʽΪ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O���ʴ�Ϊ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
��3����μӷ�Ӧ����������Ϊm��
����ԭ��HNO3�����ʵ���Ϊn
�� 3Fe+8HNO3=3Fe��NO3��2+2NO��+4H2O
3��56g 2mol������ԭ�� 2��22.4L
m n 11.2L
�� m=
| 11.2L��3��56g |
| 2��22.4L |
n=
| 11.2L��2mol |
| 2��22.4L |
�𣺲μӷ�Ӧ����������42g������ԭ����������ʵ�����0.5mol��

��ϰ��ϵ�д�
�����Ŀ