��Ŀ����
����Ŀ��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ |
|
| HClO |
|
|
����ƽ�ⳣ�� |
|
|
|
|
|
��1��25��ʱ����Ũ�ȵ�![]() ��Һ��
��Һ��![]() ��Һ��
��Һ��![]() ��Һ��3����Һ��pH�ɴ�С��˳��Ϊ________��
��Һ��3����Һ��pH�ɴ�С��˳��Ϊ________��
��2����ҵ�Ͽ��ð�ˮ��ȥβ��![]() ����
����![]() ͨ�백ˮ�У���
ͨ�백ˮ�У���![]() ����
����![]() _____��
_____��
��3�������£���![]() ��Һ�ζ�
��Һ�ζ�![]() ��Һ���õζ�������ͼ��
��Һ���õζ�������ͼ��
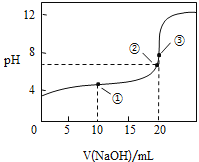
��������ʵ������У�����Ҫ����������Ʒ�� ______������ţ���
![]() ����ƿ b ��ƿc �ζ��ܼ�d ©��e ������f �ζ���
����ƿ b ��ƿc �ζ��ܼ�d ©��e ������f �ζ���
�ڵ���ζ��յ�ı�־�� _____________ ��
�����в����ᵼ�²ⶨ���ƫ�ߵ��� ______ ��
A ��ʽ�ζ�����װҺǰδ�ñ�NaOH��Һ��ϴ
B �ζ������У���ƿҡ����̫���ң���ƿ����Һ�ν���
C ��ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��յ�ʱ��������
D �ﵽ�ζ��յ�ʱ�����Ӷ���
����ͼ�����ʾ��Һ��![]() __________
__________![]() ������������������=������ͬ�������ʾ��Һ�У�
������������������=������ͬ�������ʾ��Һ�У�![]() ________
________![]() �������ʾ��Һ����������Ũ���ɴ�С��˳��Ϊ��_________��
�������ʾ��Һ����������Ũ���ɴ�С��˳��Ϊ��_________��
���𰸡�Na2CO3��Һ��Na2SO3��Һ��CH3COONa��Һ 0.62 ade �������һ���������ƣ���Һ��Ϊ��ɫ����30s�ڲ���ɫ AD �� �� ![]()
��������
(1)��ĵ���ƽ�ⳣ��Խ�����Ӧ���������ˮ��̶�ԽС����Ũ�ȵ�������Һ��pHԽС��
(2) ![]() =
= ![]() ��
��![]() =
=![]() �������㣻
�������㣻
(3)������к͵ζ�ʵ������Ҫ������Ϊ��ʽ�ζ��ܡ���ʽ�ζ��ܡ��ζ��ܼС�����̨����ͷ�ιܡ��ձ�����ƿ����������NaOH��Һ�Թ���ʱ����̪��Ϊdz��ɫ���۸���c(����)= ![]() ��������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ�������ͼ��֪�������ʾ��ҺΪ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ����Һ����Һ�����ԣ���c(H+)��c(OH-)��������ӹ�ϵΪc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)������𣻵����ʾ��Һ�����ԣ���c(H+)=c(OH-)�����ݵ�ɹ�ϵ������𣻵��ǡ�����ɴ����ƣ���������ˮ���ص�����жϡ�
��������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ�������ͼ��֪�������ʾ��ҺΪ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ����Һ����Һ�����ԣ���c(H+)��c(OH-)��������ӹ�ϵΪc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)������𣻵����ʾ��Һ�����ԣ���c(H+)=c(OH-)�����ݵ�ɹ�ϵ������𣻵��ǡ�����ɴ����ƣ���������ˮ���ص�����жϡ�
(1)��ĵ���ƽ�ⳣ��Խ�����Ӧ���������ˮ��̶�ԽС����Ũ�ȵ�������Һ��pHԽС������ƽ�ⳣ����CH3COOH��HSO3-��HCO3-����ˮ��̶ȣ�CH3COO-��SO32-��CO32-��ˮ��̶�Խ������ͬŨ�ȵ�������Һ��pHԽ����3����Һ��pH�ɴ�С��˳��Ϊ��Na2CO3��Һ��Na2SO3��Һ��CH3COONa��Һ���ʴ�Ϊ��Na2CO3��Һ��Na2SO3��Һ��CH3COONa��Һ��
(2)![]() =
= ![]() ��
��![]() =
=![]() =
=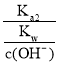 =
=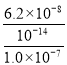 =0.62���ʴ�Ϊ��0.62��
=0.62���ʴ�Ϊ��0.62��
(3)������к͵ζ�ʵ���������Ҫ��ʽ�ζ��ܡ���ʽ�ζ��ܡ��ζ��ܼС�����̨���ձ�����ƿ������������Ҫ����ƿ��©�������������ʴ�Ϊ��ade��
��NaOH��Һ�Թ���ʱ����̪��Ϊdz��ɫ������NaOH��Һ�ζ�CH3COOH�ﵽ�ζ��յ�ʱ���������һ��NaOH��Һ����̪��Ϊdz��ɫ������30s�ڲ���ɫ��
�ʴ�Ϊ���������һ���������ƣ���Һ��Ϊdz��ɫ����30s�ڲ���ɫ��
��A����ʽ�ζ�����װҺǰδ�ñ�NaOH��Һ��ϴ���������ı�Һ���ƫ�ߣ�����c(����)= ![]() ������֪������ҺŨ��ƫ�ߣ���Aѡ��B���ζ������У���ƿҡ����̫���ң���ƿ����Һ�ν������������ı�Һ���ƫ�ͣ�����c(����)=
������֪������ҺŨ��ƫ�ߣ���Aѡ��B���ζ������У���ƿҡ����̫���ң���ƿ����Һ�ν������������ı�Һ���ƫ�ͣ�����c(����)= ![]() ������֪������ҺŨ��ƫ�ͣ���B��ѡ��C����ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��յ�ʱ�������ݣ��������ı�Һ�������ƫ�ͣ�����c(����)=
������֪������ҺŨ��ƫ�ͣ���B��ѡ��C����ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��յ�ʱ�������ݣ��������ı�Һ�������ƫ�ͣ�����c(����)= ![]() ������֪������ҺŨ��ƫ�ͣ���C��ѡ��D���ﵽ�ζ��յ�ʱ�����Ӷ������������ı�Һ�������ƫ�ߣ�����c(����)=
������֪������ҺŨ��ƫ�ͣ���C��ѡ��D���ﵽ�ζ��յ�ʱ�����Ӷ������������ı�Һ�������ƫ�ߣ�����c(����)= ![]() ������֪������ҺŨ��ƫ�ߣ���Dѡ���ʴ�Ϊ��AD��
������֪������ҺŨ��ƫ�ߣ���Dѡ���ʴ�Ϊ��AD��
����ͼ��֪�������ʾ��ҺΪ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ����Һ����Һ�����ԣ���c(H+)��c(OH-)����ʱ���ӹ�ϵΪc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)����c( CH3COO-)+c(OH-)=c(CH3COOH)+c(H+)+[(H+)-c(OH-)]��c(CH3COOH)+c(H+)�������ʾ��Һ�����ԣ���c(H+)=c(OH-)����ɹ�ϵΪc( CH3COO-)+c(OH-)=c(Na+)+c(H+)����c(Na+)=c( CH3COO-)������Һ�л�����CH3COOH������c(Na+)��c( CH3COO-)+c(CH3COOH)�����ǡ�����ɴ����ƣ���������ˮ���ص��֪��![]() ���ʴ�Ϊ����������
���ʴ�Ϊ����������![]() ��
��