��Ŀ����
��ͼ��ʾ��ѧ��Ӧ�����е������仯����ͼ�ж�����˵���к������ǣ� ��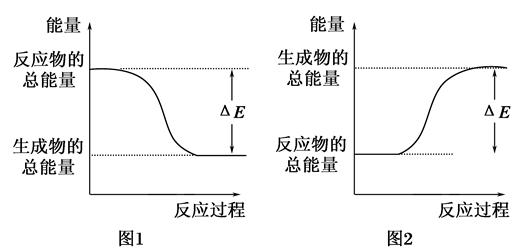
| A��500mL 2.0mol��L��1 HCl��500mL 2.0mol��L��1 NaOH�ķ�Ӧ����ͼ1���Ҧ�E��57.3kJ |
| B��500mL 2.0mol��L��1 H2SO4��500mL 2.0mol��L��1 Ba��OH��2�ķ�Ӧ����ͼ2���Ҧ�E��114.6kJ |
| C������ͼ1�����仯���κη�Ӧ��һ��������ȼ��ɷ��� |
| D��CaO��Ũ��������ˮʱ�������仯����ͼ1 |
A
����

��ϰ��ϵ�д�
�����Ŀ
��֪��2H2(g)��O2(g)��2H2O(l) ��H����571.6 kJ��mol��1
2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(l) ��H����1452 kJ��mol��1
H��(aq)��OH��(aq)��H2O(l) ��H����57.3 kJ��mol��1
����˵����ȷ���ǣ� ��
| A��H2(g)��ȼ����Ϊ -571.6 kJ��mol��1 |
| B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������� |
C��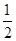 H2SO4(aq)�� H2SO4(aq)��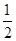 Ba(OH)2(aq)�� Ba(OH)2(aq)�� 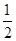 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 |
| D��3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����135.9 kJ��mol��1 |
������ͼ�������仯ʾ��ͼ������ѡ����ȷ����
| A��2A+B�T2C����H��O |
| B��2C�T2A+B����H��0 |
| C��2A(g��+B(g���T2C(g����H��0 |
| D��2A(g��+B(g���T2C(g����H��0 |
��֪��ӦA+B=C+DΪ���ȷ�Ӧ,�Ը÷�Ӧ������˵����ȷ����( )��
| A��A������һ������C |
| B��B������һ������D |
| C��A��B��������һ������C��D�������� |
| D���÷�ӦΪ���ȷ�Ӧ,�ʲ��ؼ��Ⱦ�һ���ܷ��� |
�˹���������ܹ�����̫���ܣ���CO2��H2O�Ʊ���ѧԭ�ϡ���ͼ��ͨ���˹���������Ʊ�HCOOH��ԭ��ʾ��ͼ������˵������ȷ���� (����)
| A���ù����ǽ�̫����ת��Ϊ��ѧ�ܵĹ��� |
| B������a���淢��������Ӧ����O2���� |
| C������a�������Լ���������b����������ǿ |
| D������b����ķ�Ӧ��CO2��2H����2e��=HCOOH |
������H2SO4��Һ�м���110 mL 0.4 mol/L Ba(OH)2��Һ���ų���������5.12 kJ�����������Ba(OH)2��Һ�м���110 mL 0.4 mol/L HCl��Һʱ���ų���������2.2 kJ����Na2SO4��Һ��BaCl2��Һ��Ӧ���Ȼ�ѧ����ʽΪ(����)
| A��Ba2��(aq)��SO42��(aq)=BaSO4(s)��H����0.72 kJ/mol |
| B��Ba2��(aq)��SO42��(aq)=BaSO4(s)��H����2.92 kJ/mol |
| C��Ba2��(aq)��SO42��(aq)=BaSO4(s)��H����16.4 kJ/mol |
| D��Ba2��(aq)��SO42��(aq)=BaSO4(s)��H����73.0 kJ/mol |
�����Ȼ�ѧ����ʽ��,��ȷ����(����)
A�������ȼ���Ȧ�HΪ-890.3 kJ��mol-1,�����ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ:CH4(g)+2O2(g) CO2(g)+2H2O(g)����H="-890.3" kJ��mol-1 CO2(g)+2H2O(g)����H="-890.3" kJ��mol-1 |
B����20.0 g��NaOH��ϡ��Һ��ϡ������ȫ�к�,�ų�28.7 kJ ������,��ϡ�����ϡNaOH��Һ��Ӧ���Ȼ�ѧ����ʽΪ:NaOH(aq)+CH3COOH(aq) CH3COONa(aq)+H2O(l)����H="-57.4" kJ��mol-1 CH3COONa(aq)+H2O(l)����H="-57.4" kJ��mol-1 |
C����ͼ��298 K��101 PaʱCO��H2�ϳ�CH3OH(g)�ķ�Ӧ�����������仯������ͼ,��÷�Ӧ���Ȼ�ѧ����ʽΪ:CO(g)+2H2(g) CH3OH(g)����H="+91" kJ��mol-1 CH3OH(g)����H="+91" kJ��mol-1 |
D����֪:2Zn(s)+O2(g) 2ZnO(s) ��H="-701.0" kJ/mol 2ZnO(s) ��H="-701.0" kJ/mol |
 2HgO(s) ����H="-181.6" kJ/mol
2HgO(s) ����H="-181.6" kJ/mol��Zn(s)+HgO(s)
 ZnO(s)+Hg(l) ��H="-259.7" kJ��mol-1
ZnO(s)+Hg(l) ��H="-259.7" kJ��mol-1 ��һ��������A��B��Ӧ������C��D���������仯��ͼ��
�����йط�ӦA��B=C��D��˵����ȷ���� ����������
| A����Ӧǰ��ԭ�ӵ��������Ŀһ������ |
| B����Ӧǰ����ӵ��������Ŀһ���ı� |
| C����Ӧ���������E1���������������E2һ����� |
| D���˷�Ӧһ���������ı仯 |

 N2O4(g)�У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬
N2O4(g)�У������ʵ�Ũ��������������֮��Ĺ�ϵ�����н���A��Ӧ��״̬Ϊ��ѧƽ��״̬