��Ŀ����
��֪��2H2(g)��O2(g)��2H2O(l) ��H����571.6 kJ��mol��1
2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(l) ��H����1452 kJ��mol��1
H��(aq)��OH��(aq)��H2O(l) ��H����57.3 kJ��mol��1
����˵����ȷ���ǣ� ��
| A��H2(g)��ȼ����Ϊ -571.6 kJ��mol��1 |
| B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������� |
C�� H2SO4(aq)�� H2SO4(aq)�� Ba(OH)2(aq)�� Ba(OH)2(aq)��  BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 |
| D��3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����135.9 kJ��mol��1 |
B
�������������A. ȼ������1mol��������ȫȼ�ղ����ȶ���������ʱ���ų�������������ʽ�и�������2mol��������ȫȼ�����ų�������������A.�ɷ���ʽ��֪1gH2ȼ�շų�����142.9KJ/g, 1gCH3OH(l)��ȫȼ�շų�����1452 kJ��32=45.375KJ/g����ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������ࡣ��ȷ��C���ڷ�Ӧ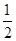 H2SO4(aq)��
H2SO4(aq)��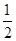 Ba(OH)2(aq)��
Ba(OH)2(aq)�� 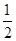 BaSO4(s)��H2O(l)�г���H+��OH-��Ӧ����ˮ�����г��������ų��������Ը�ԭ��Ҫ��57.3 kJ��mol��1�ࡣ����C��
BaSO4(s)��H2O(l)�г���H+��OH-��Ӧ����ˮ�����г��������ų��������Ը�ԭ��Ҫ��57.3 kJ��mol��1�ࡣ����C��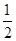 H2SO4(aq)��
H2SO4(aq)�� Ba(OH)2(aq)��
Ba(OH)2(aq)��  BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1D.�����١�3���ڣ���2�����ɵ�3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����131.4kJ��mol��1.����
BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1D.�����١�3���ڣ���2�����ɵ�3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����131.4kJ��mol��1.����
���㣺�����˹���ɼ�Ӧ�õ�֪ʶ��

������������ϵ����ò���ȷ����
| A��������ЧӦ��С�����ж�ijЩ���ʵ��ȶ���ǿ�� |
| B�������ܶ����ݿ��ж�Һ�������Ƿ����ͨ��������� |
| C������ԭ��(������)�뾶���ݿ��ƶ�ijЩԭ��(������)�������Ժͻ�ԭ�Ե�ǿ�� |
| D�������ܽ�����ݿ��Ʋ⽫һЩ���ʻ������뿪���Ŀ����� |
��������ȫ�������ů�����������ͷ�չ�������Ͼ�����ս���ڴ˱����£�������Դ��������̼���������ܼ��š������Ը�ե�����ȸ����������ܵ����ǵ����ӡ������й�˵������ȷ���ǣ� ��
| A��̫���ܡ������ܡ��������ܺͺ˾۱��ܾ����ڡ�����Դ�� |
| B������̼����ָ���ú�̼���͵�������Ϊȼ�� |
C����ͼ���龭һ�ȼ������ɵ�̼ϩ����;�������ˡ����ܼ��š�˼��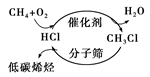 |
| D����ú��ɺϳ�������ú���Ը�ե������ʵ����ú����ࡢ��Ч���� |
���з�Ӧһ�����ڷ��ȷ�Ӧ����(����)��
| A����������������Ȼ�茶���ķ�Ӧ |
B�������仯��ͼ��ʾ�ķ�Ӧ |
| C����ѧ���������յ������Ȼ�ѧ���γɷų��������ٵķ�Ӧ |
| D������Ҫ���Ⱦ��ܷ����ķ�Ӧ |
��ͼ��ʾ��ѧ��Ӧ�����е������仯����ͼ�ж�����˵���к������ǣ� ��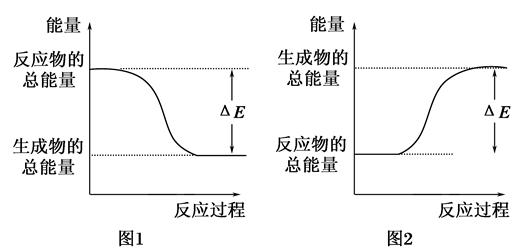
| A��500mL 2.0mol��L��1 HCl��500mL 2.0mol��L��1 NaOH�ķ�Ӧ����ͼ1���Ҧ�E��57.3kJ |
| B��500mL 2.0mol��L��1 H2SO4��500mL 2.0mol��L��1 Ba��OH��2�ķ�Ӧ����ͼ2���Ҧ�E��114.6kJ |
| C������ͼ1�����仯���κη�Ӧ��һ��������ȼ��ɷ��� |
| D��CaO��Ũ��������ˮʱ�������仯����ͼ1 |
����ij������˾�������������������ڽϵ��¶��½�����ת������ϩ��2CH4(g)??C2H4(g)��2H2(g)����H����֪�ڽ����¶�ʱ�÷�Ӧƽ�������ƶ�����������������Ӧ(Q1��Q2��Ϊ��ֵ)��
��Ӧ��C(s)��2H2(g)=CH4(g)����H1����Q1
��Ӧ��C(s)��H2(g)= C2H4(g)����H2����Q2
C2H4(g)����H2����Q2
�������ж���ȷ����(����)
| A����H��0 | B��Q2��Q1 | C����H��2(Q1��Q2) | D����H��Q1��Q2 |
��֪��
S(s)��O2(g) SO2(g)����H����297.16 kJ��mol��1��
SO2(g)����H����297.16 kJ��mol��1��
2SO2(g)��O2(g) 2SO3(g)����H����196.6 kJ��mol��1��
2SO3(g)����H����196.6 kJ��mol��1��
����˵����ȷ���� (����)
| A��1 mol SO2(g)�������ܺʹ���1 mol S(s)��1 mol O2(g)�������ܺ� |
| B����2 mol SO2(g)��1 mol O2(g)��һ�������³�ַ�Ӧ���ų�196.6 kJ������ |
| C��S(g)��O2(g)=SO2(g)����H����Q��QֵС��297.16 kJ |
| D����1 mol S(s)��ȫת��ΪSO3(g)ʱ(������������ʧ)���ų�395.46 kJ������ |