��Ŀ����
8����ά����C��C6H8O6����Ҫ�������߲ˡ�ˮ���У�������ǿ����Լ����ĵֿ���������1��ȡ��Ƭά����C��ѹ�������100mLˮ�й��ˣ�ȡ������Һ�����еμӼ�����ɫʯ����Һ����Һ��죬˵��ά����C��ˮ��Һ�����ԣ�
��2��ά����C�����Ի����бȽ��ȶ����ڸ���ʱ�ױ��ƻ���������һ�룬���ʳ�ûƹϣ����ܳ�����ûƹ��зḻ��ά����C��ʳ�ûƹ�ʱ��üӵ�����裮
��ij����ʳƷ�����װ��ǩ�ϵIJ����������£�
| ��Ҫԭ�ϣ�̼��� ʳ�÷�����ÿ��һ�Σ�ÿ��һƬ����ʳ�� |
��2�����úñ���ʳƷ�е�̼�����θҺ�е����ᷢ����Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3��ʳ�÷����н�ʳ������������Ӧ��ĽӴ�������ٽ��Ƶ����գ�
���� ��1������ȡ������Һ�����еμӼ�����ɫʯ����Һ����Һ��죬��֪ά����C����Һ�����ԣ���2���������Ի����бȽ��ȶ����ڸ���ʱ���ױ��ƻ������ʣ���֪�����������¶ȵ��ܳ�����ûƹ��зḻ��ά����C��
��1���Ķ���ǩ�����ݱ���ʳƷ�ijɷַ�������ʳƷ����Ҫ���ܣ�
��2������̼�����θҺ�е����ᷴӦ�ķ�Ӧ�P������д����Ӧ�ķ���ʽ��
��3��ͨ����ʳ�ɽ��ϴ�Ƭ״��ʳƷ���ϸС�Ŀ�����
��� �⣺��1����������ʯ���죬������Ϣ��֪ά����C����Һ�����ԣ��ʴ�Ϊ���
��2����ά����C�����ȶ������±��ƻ����ʴ�Ϊ��ʳ�ûƹ�ʱ��üӵ�����裻
��1��ͨ���Ķ���ǩ��֪������ʳƷ����Ҫԭ����̼��ƣ�ͨ��ʳ�ã������������ڲ���Ԫ�أ��ʴ�Ϊ�����ƣ�
��2��̼�����θҺ�е����ᷢ����Ӧ���������Ȼ��ơ�ˮ��������̼�����������غ㶨�ɣ�����д����Ӧ�ķ���ʽ��CaCO3+2HCl=CaCl2+H2O+CO2�����ʴ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3��ͨ����ʳ�ɽ��ϴ�Ƭ״��ʳƷ���ϸС�Ŀ���������Ӧ��ĽӴ�������ٽ��Ƶ����գ��ʴ�Ϊ������Ӧ��ĽӴ�������ٽ��Ƶ����գ�
���� ������Ҫ����ά����C��̼��Ƶ�֪ʶ���ѶȲ�����ʱҪ�����Ķ���ǩ����ȡ������Ϣ��������������⣮

 ������������ϵ�д�
������������ϵ�д�| A�� | ���Ȼ����ʱ��ŨHNO3�������ֽ� | |
| B�� | �Ӵ�����ʹ������������һ��������ת��Ϊ���� | |
| C�� | ����Ũ��ˮ���������ƹ��������ȡ���� | |
| D�� | H2��I2��HIƽ��������ѹ����ɫ���� |
 ��ͼ��ʾ������ƿ����ˮ����Һ©���ڵ�Һ��Ҳ��ˮ�����ձ��ڵμ�ˮʱ������U�ι���Һ���������ƣ��ָ���ԭ�¶Ⱥ�Һ��������ұ�����ƽ�����ձ��ڵ������ǣ�������
��ͼ��ʾ������ƿ����ˮ����Һ©���ڵ�Һ��Ҳ��ˮ�����ձ��ڵμ�ˮʱ������U�ι���Һ���������ƣ��ָ���ԭ�¶Ⱥ�Һ��������ұ�����ƽ�����ձ��ڵ������ǣ�������| A�� | �������� | B�� | ������ | C�� | �� | D�� | �� |
 ��ͼ��ʾ���Թܢ���ʢ��98���ˮ���Թܢ��г�������B����Һ��A�����Թܢ��У���ַ�Ӧ�����K�������Թܢ��е�ˮ���̷��ڣ���A��B�����ǣ�������
��ͼ��ʾ���Թܢ���ʢ��98���ˮ���Թܢ��г�������B����Һ��A�����Թܢ��У���ַ�Ӧ�����K�������Թܢ��е�ˮ���̷��ڣ���A��B�����ǣ�������| A�� | �������� | B�� | ϡ������һ����̼ | ||
| C�� | ����ʳ��ˮ������ | D�� | Ũ��ˮ����ϩ |
 ͭ���仯�����ڹ�ҵ��ũҵ���Ƽ����ճ��������й㷺Ӧ�ã�
ͭ���仯�����ڹ�ҵ��ũҵ���Ƽ����ճ��������й㷺Ӧ�ã� ����������[CH3CH��OH��COO]2Fe•3H2O��Mr=288����һ�ֳ��õIJ���������ͨ��������̼��������Ӧ�Ƶã�
����������[CH3CH��OH��COO]2Fe•3H2O��Mr=288����һ�ֳ��õIJ���������ͨ��������̼��������Ӧ�Ƶã�
 ���÷��ӵĺ˴Ź���������2�ַ壮
���÷��ӵĺ˴Ź���������2�ַ壮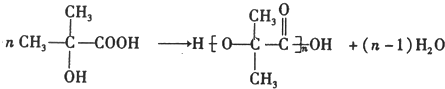 ��F��G�ķ�Ӧ����CH3��2C��OH��-CHO+2Cu��OH��2$\stackrel{��}{��}$��CH3��2C��OH��-COOH+Cu2O��+2H2O��
��F��G�ķ�Ӧ����CH3��2C��OH��-CHO+2Cu��OH��2$\stackrel{��}{��}$��CH3��2C��OH��-COOH+Cu2O��+2H2O�� ��S2-
��S2-
