��Ŀ����
��֪A��F����ѧ��ѧ�г������ʣ�����A��C��E��FΪ���壬B��DΪҺ�壬D������������Ϊһ�����ҹ�ҵ����ˮƽ��һ�ֱ�־��F��Ũ��Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӡ���Ӧ�в�������������ȥ��
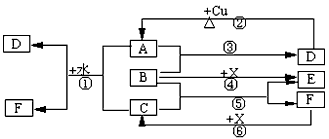
�Իش����У�
��1������ͼ����Ϣ��B��C��D��X�����Դ�ǿ������˳����____________________��
��2��B�ĵ���ʽΪ��____________________��
��3��д����Ӧ�ڵĻ�ѧ����ʽ��________________________________________��
��4��д����Ӧ�١������ӷ���ʽ
��_________________________________________________ ��
��__________________________________________________��
��1������ͼ����Ϣ��B��C��D��X�����Դ�ǿ������˳����____________________��
��2��B�ĵ���ʽΪ��____________________��
��3��д����Ӧ�ڵĻ�ѧ����ʽ��________________________________________��
��4��д����Ӧ�١������ӷ���ʽ
��_________________________________________________ ��
��__________________________________________________��
��1��X��C��B��D�����MnO2 ��Cl2�� H2O2 ��H2SO4��
��2��
��3��Cu +2H2SO4(Ũ) CuSO4 +SO2 ��+2H2O
CuSO4 +SO2 ��+2H2O
��4��Cl2 +SO2 +2H2O = 4H+ + SO42��+2Cl��
MnO2+4H+ + 2Cl�� Mn2+ +Cl2 ��+2H2O
Mn2+ +Cl2 ��+2H2O
��2��

��3��Cu +2H2SO4(Ũ)
 CuSO4 +SO2 ��+2H2O
CuSO4 +SO2 ��+2H2O ��4��Cl2 +SO2 +2H2O = 4H+ + SO42��+2Cl��
MnO2+4H+ + 2Cl��
 Mn2+ +Cl2 ��+2H2O
Mn2+ +Cl2 ��+2H2O 
��ϰ��ϵ�д�
�����Ŀ
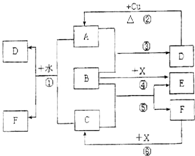 ��֪A��F����ѧ��ѧ�г������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺
��֪A��F����ѧ��ѧ�г������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺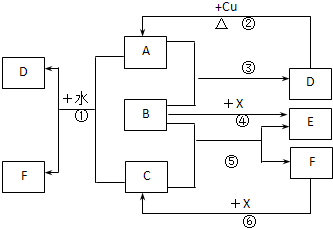
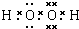

 ��֪A��F����ѧ��ѧ���������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺
��֪A��F����ѧ��ѧ���������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺