��Ŀ����
18��������ˮ����Cl2��H2O��HClO��H+��Cl-�����ӣ�Ϊ������ɷ֣�ij�о���ѧϰС����������ʵ�飬���������ʵ�飬��Ҫ����գ���1��ȡ����������ˮ���Թ��У�����̼���Ʒ�ĩ�����������ݲ�������˵�������õijɷ���H+��
��2��ȡ����������ˮ���Թ��У�����AgNO3��Һ�������а�ɫ�����������������õ�����Cl-��
��3������ɫʯ����ֽ�μӼ���������ˮ����ֽ�ȱ������Ϊ����H+�����ܿ�����ɫ����Ϊ����HClO��
��4�����Ƶ���ˮ�ʵ�����ɫ��˵����ˮ����Cl2���Ӵ��ڣ�
���� ��������ˮ������ӦCl2+H2O?HCl+HClO��������ˮ����Cl2��H2O��HClO��H+��Cl-�����ӣ�����H+����̼���Ƶ����ʷ�Ӧ����Cl2��HClO����ǿ�����ԣ�HClO����Ư���ԣ���Һ�к���Cl-������������Ӧ���ɰ�ɫAgCl�������Դ˽����⣮
��� �⣺��1�������̼��Ʒ�Ӧ�����Ȼ��ƺͶ�����̼���壬HCl���ֳ����ԣ��ʴ�Ϊ��H+��
��2����Һ�к��У�Cl-������������Ӧ���ɰ�ɫAgCl�������ʴ�Ϊ��Cl-��
��3����ֽ�ȱ������Ϊ����H+����Ϊ����HClO����Ư���ԣ���ʹ��ɫ������ɫ���ʴ�Ϊ��H+��HClO��
��4�����Ƶ���ˮ�ʵ�����ɫ��˵����ˮ����Cl2���ʴ�Ϊ��Cl2��
���� �����ۺϿ�����������ˮ�����ʣ�ע�����������ˮ��Ӧ���ص��Լ���ˮ�ijɷֺ����ʣ�Ϊ�߿���Ƶ���㣬�����ڻ���֪ʶ���ۺ����ã��ѶȲ���

��ϰ��ϵ�д�
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
6������ϩ�ǻ�����Ʒ������Ҫ�ĵ���֮һ���ڹ�ҵ�ϣ�
����ϩ���ұ���CO2�������Ƶã��ܷ�Ӧԭ�����£�
 ��g��+CO2��g��?
��g��+CO2��g��? ��g��+CO��g��+H2O��g����H
��g��+CO��g��+H2O��g����H
�ش��������⣺
��1����֪��
���ұ���ȡ����ϩ��Ӧ�ġ�H=+158.8KJ/mol
��2�����¶�ΪT1ʱ���÷�Ӧ��ƽ�ⳣ��K=0.5mol/L����2L���ܱ������м����ұ���CO2����Ӧ��ijʱ�̲�øû�������ֵ����ʵ�����Ϊ1.0mol��
�ٸ�ʱ�̻�ѧ��Ӧ�ǣ���ǡ����ǡ�������ƽ��״̬��
������������˵���ұ���C02�ڸ���������ƽ��״̬����AD������ȷ�𰸱�ţ���
A�������淴Ӧ���ʵı�ֵ�㶨 B��c��CO2��=c��CO��
C�����������ܶȲ��� D��CO2������������ֲ���
��������Ӧ��Ϊ��ѹ���������½��У��ﵽƽ��ʱ�����ұ������ʵ���Ũ��d������ȷ�𰸱�ţ���
a������0.5mol/L��������b��С��0.5mol/L�������� c������0.5mol/Ld����ȷ��
��3�����¶�ΪT2ʱ�ĺ㶨�����У��ұ���CO2����ʼŨ�ȷֱ�Ϊ1.0mol/L��2.0mol/L���跴Ӧƽ�����ѹǿΪP����ʼѹǿΪP0����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ$\frac{5��P-{P}_{0}��}{{P}_{0}}$���ұ���ת����Ϊ$\frac{2.5��P-{P}_{0}��}{{P}_{0}}$��100%�����ú�P0��P�ı���ʽ��ʾ����
��4��д���ɱ���ϩ��һ�������ºϳɾ۱���ϩ�Ļ�ѧ����ʽ ��
��
����ϩ���ұ���CO2�������Ƶã��ܷ�Ӧԭ�����£�
 ��g��+CO2��g��?
��g��+CO2��g��? ��g��+CO��g��+H2O��g����H
��g��+CO��g��+H2O��g����H�ش��������⣺
��1����֪��
| ��ѧ�� | C-H | C-C | C=C | C=O | CO | O-H |
| ����/kJ/mol | 413 | 348 | 615 | 745 | 1076 | 463 |
��2�����¶�ΪT1ʱ���÷�Ӧ��ƽ�ⳣ��K=0.5mol/L����2L���ܱ������м����ұ���CO2����Ӧ��ijʱ�̲�øû�������ֵ����ʵ�����Ϊ1.0mol��
�ٸ�ʱ�̻�ѧ��Ӧ�ǣ���ǡ����ǡ�������ƽ��״̬��
������������˵���ұ���C02�ڸ���������ƽ��״̬����AD������ȷ�𰸱�ţ���
A�������淴Ӧ���ʵı�ֵ�㶨 B��c��CO2��=c��CO��
C�����������ܶȲ��� D��CO2������������ֲ���
��������Ӧ��Ϊ��ѹ���������½��У��ﵽƽ��ʱ�����ұ������ʵ���Ũ��d������ȷ�𰸱�ţ���
a������0.5mol/L��������b��С��0.5mol/L�������� c������0.5mol/Ld����ȷ��
��3�����¶�ΪT2ʱ�ĺ㶨�����У��ұ���CO2����ʼŨ�ȷֱ�Ϊ1.0mol/L��2.0mol/L���跴Ӧƽ�����ѹǿΪP����ʼѹǿΪP0����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ$\frac{5��P-{P}_{0}��}{{P}_{0}}$���ұ���ת����Ϊ$\frac{2.5��P-{P}_{0}��}{{P}_{0}}$��100%�����ú�P0��P�ı���ʽ��ʾ����
��4��д���ɱ���ϩ��һ�������ºϳɾ۱���ϩ�Ļ�ѧ����ʽ
 ��
��
13������˵���д�����ǣ�������
| A�� | ԭ�Ӽ������ӵĺ�����Ӳ������ڸ�Ԫ�����ڵ������� | |
| B�� | Ԫ�����ڱ��д�IIIB�嵽IIB�� 10�����е�Ԫ�ض��ǽ���Ԫ�� | |
| C�� | �������ϡ������ԭ�ӵ���������������8 | |
| D�� | ͬһԪ�صĸ���ͬλ�ص��������ʡ���ѧ���ʾ���ͬ |
8�� ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ��������������£�
ʵ�鲽�����£�
�ٽ�6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У�
����A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֣�
�۽�����ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��B�����������Ƿ�Һ©����D�����������������ܣ�
��2��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�Һʱ��ˮ���²㣨��ϡ����¡���
��3����Ӧ�¶�Ӧ������90��95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ�����������¶ȹ��߱�����Ϊ���ᣩ��
��4����ʵ���У�����ȩ�IJ���Ϊ51.5%����������λ��Ч���֣�
 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO��Ӧ��Ͳ��������������£�
| �е�/�� | �ܶȣ�g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.5 | 0.8107 | �� |
�ٽ�6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У�
����A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֣�
�۽�����ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��B�����������Ƿ�Һ©����D�����������������ܣ�
��2��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�Һʱ��ˮ���²㣨��ϡ����¡���
��3����Ӧ�¶�Ӧ������90��95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ�����������¶ȹ��߱�����Ϊ���ᣩ��
��4����ʵ���У�����ȩ�IJ���Ϊ51.5%����������λ��Ч���֣�
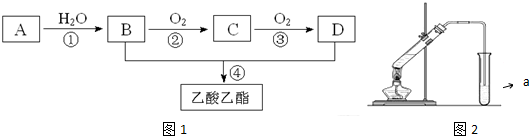
 ��C�Ľṹ��ʽ��CH3CHO��
��C�Ľṹ��ʽ��CH3CHO��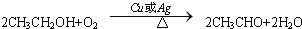 ����Ӧ���ͣ�������Ӧ��
����Ӧ���ͣ�������Ӧ��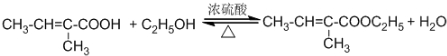 ��Ϊ�Ӹ�ʵ���Ļ�������з���������������Ҳ��Թ�����ѡ�õ��Լ�a�DZ���̼������Һ��a�Լ����������к����ᡢ�����Ҵ�������������������Һ�е��ܽ�ȣ������ڷֲ�������
��Ϊ�Ӹ�ʵ���Ļ�������з���������������Ҳ��Թ�����ѡ�õ��Լ�a�DZ���̼������Һ��a�Լ����������к����ᡢ�����Ҵ�������������������Һ�е��ܽ�ȣ������ڷֲ�������

