��Ŀ����
ijͬѧ����ԭ���ԭ����ʵ��ʱ,������ʵ�鲽��:
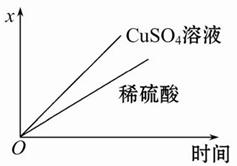
���õ��߽����������Ƶ����˷ֱ��봿����пƬ��ͭƬ������(��ͼ1);
�ڰ�һ�鴿����пƬ����ʢ��ϡ������ձ���;
�۰�һ�鴿����ͭƬ����ʢ��ϡ������ձ���;
���õ��߰�пƬ��ͭƬ����������,��ƽ�еز���ʢ��ϡ������ձ���(��ͼ2)��
�ش���������:
(1)ʵ�鲽�����Ӧ�۲쵽���������� ��
(2)ʵ�鲽�����Ӧ�۲쵽���������� ��
(3)ʵ�鲽�����Ӧ�۲쵽���������� ��
(4)ʵ�鲽�����Ӧ�۲쵽���������� ��
(5)ͨ��ʵ�鲽��ܸ�ͬѧͷ��������һ������(�����),�ò���������
(6)Ϊ��֤ʵ�ò���,��ͬѧ������˵ڢݲ�ʵ��,���Ҫ�����ڢݲ�ʵ���װ��ʾ��ͼ��
(1)������ָ�벻ƫת
(2)пƬ�������ݲ���
(3)ͭƬ��������
(4)ͭƬ���д�������,пƬ��û�����ݻ�����������
(5)�е��Ӵ�п��������ͭƬ�ƶ�
(6)�е��Ӵӵ���������,������Ӧ�γɵ���,��������һ����������֤��,ʵ��װ��ͼ���������,��ҺΪϡ���ᡣ
����

 ��У����ϵ�д�
��У����ϵ�д���6��)ZnMnO2�ɵ��Ӧ�ù㷺����������Һ��ZnCl2NH4Cl�����Һ��
��1���õ�صĸ���������________����ع���ʱ����������________(��������� �� )��
��2����ZnCl2NH4Cl�����Һ�к�������Cu2���������ij�缫�ĸ�ʴ������Ҫԭ����____________��
����ȥCu2�������ѡ�������Լ��е�________(�����)��
| A��NaOH | B��Zn | C��Fe | D��NH3��H2O |

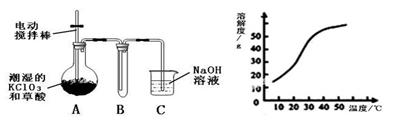
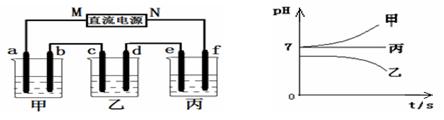



 2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣