��Ŀ����
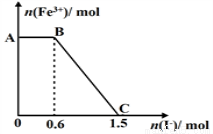
��6�֣�ij����NH4HCO3�л�������(NH4)2CO3���ֲ������з����ⶨ�õ�����(NH4)2CO3��������������ȡ5.7 g������Ʒ��2.0 mol/L NaOH��Һ��ϣ���ȫ�ܽ���¼���ʹ���ַ�Ӧ(���¶�����β��ֽ�)����ʹ���ɵİ���ȫ�����������գ���ð���������������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ��

��1��A��ǰ��Ʒ��NaOH��Ӧ�����ӷ���ʽΪ__________________________________
��2��Ϊʹ���ɵİ�������������ʱ����������������ѡ������װ���е�________��

��3����Ʒ��(NH4)2CO3������������________%(����һλС��)��
��1��HCO3����OH��===CO32����H2O ��2��ABD ��3��16.8
��������
�����������ͼ���֪��A��ǰ��Ϊ����NH3��������ӦΪHCO3����OH��֮��ķ�Ӧ�����ӷ���ʽΪ��HCO3����OH��=== CO32����H2O��Ϊʹ���ɵİ�������������ʱ������������������װ��Ϊ�ܷŵ�����װ�ã�ѡABD����2.0 mol/L NaOH��Һ70mlʱ����Һ����ΪNa2CO3����������NH4HCO3 xmol��(NH4)2CO3 ymol�����е�ʽ��ϵ��
��ɵã�x=0.06mol y=0.01mol���ɵ���Ʒ��(NH4)2CO3����������=
=16.8%.
���㣺ͼ��������⡣

��12�֣����������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�á���ͼK10?3��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��

ͼK10?3
��1���Ʊ�����ѡ�õ�ҩƷΪ������غ�Ũ���ᣬ��Ӧ�����ӷ���ʽΪ___________________________��
��2��װ��B��������________________________��ʵ�����ʱC�п��ܷ�����������д����������ʱB�е�����______________________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���____(ѡ��a����b����c��)��
a | b | c | |
I | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����塣��������D�е�������Һ����E�У���E���۲쵽��������________________________________��������________(��ܡ����ܡ�)˵����ķǽ�����ǿ�ڵ⡣
�����йػ�ѧ�����ʾ����ȷ����
A������ԭ�ӽṹʾ��ͼ�� |
B��Na2O2�ĵ���ʽ�� |
| C��HClO�Ľṹʽ��H��O��Cl |
D��������Ϊ16�������ӣ� S2�� S2�� |