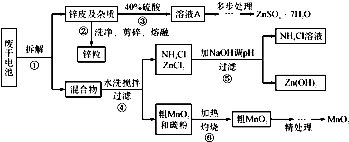
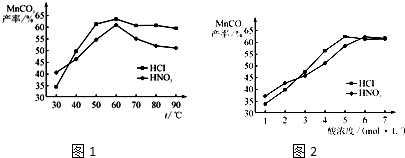
��Ŀ����
��֪
25��ʱ��A����Һ��pH��a��B����Һ��pH��b��(1)��AΪǿ�ᣬBΪǿ���a��b��14�����ߵ������Ϻ���Һ��pH��________����ʱ����Һ�м��������Ũ�ȴ������������Ũ�ȣ���ԭ�������________��
(2)��AΪǿ�ᣬBΪǿ����߰������Ϊ1��10��Ϻ���Һ�����ԣ���a��b��________��
(3)��A�Ļ�ѧʽΪHR��B�Ļ�ѧʽΪMOH����a��b��14�����ߵ������Ϻ���Һ�����ԣ�������Һ�бض���һ�������ܷ���ˮ�⣬������ˮ�ⷴӦ�����ӷ���ʽΪ________��
(4)��(3)�Ļ����Һ�У�������Ũ�ȴ�С��ϵһ����ȷ����________(�����)��
��c(MOH)��c(M+)��c(R��)��c(H+)��c(OH��)
��
c(HR)��c(M+)��c(R��)��c(OH��)��c(H+)��
c(R��)��c(M+)��c(H+)��c(OH��)��
c(M+)��c(R��)��c(OH��)��c(H+)��
c(M+)��c(H+)��c(R��)��c(OH��)
�𰸣�
������
������
|
�����𰸣� (1)7�����һԪǿ������ (2)13���� (3)R����H2O����(4)�ۢ� ���������� ���� |

��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ

 NiSO4?6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����ɵ�Ʒ������������⣬������Cu��Zn��Fe��Cr�����ʣ�Ϊԭ�ϻ�ã������������£�
NiSO4?6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����ɵ�Ʒ������������⣬������Cu��Zn��Fe��Cr�����ʣ�Ϊԭ�ϻ�ã������������£�