��Ŀ����
��10�֣���ͼ��ʾ��ӦI����ӦII�ͷ�ӦIII���ǹ�ҵ�����г����ķ�Ӧ������A��BΪ�����C����������֮һ��D��K���������������H������ΪҺ̬�����J��һ�־���Ư�����õ��Σ���ӦIII��E��G��Ӧ��ԭ����ͬ��
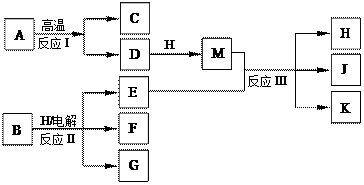
��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ�� ��
��2�� E��G��Ӧ�����ӷ���ʽ�� ��
E��G��Ӧ�����ӷ���ʽ�� ��
��3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ���� ��
������I2�ķ�Ӧ�����ӷ���ʽ�� ��

��1��C��J��ˮ��Һ��Ӧ�����ɵĺ�����ĵ���ʽ�� ��
��2��
 E��G��Ӧ�����ӷ���ʽ�� ��
E��G��Ӧ�����ӷ���ʽ�� ����3��J���ú��㲻�Ӵ�ˮ������������Ҳ�ֽ�����K�����ų����壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����ҵ�ϲⶨ��ӦIII��Ʒ����Ч�ɷ�J�ĺ������Ƚ�һ�����IJ�Ʒ����Һ���������KI��Һ��ϡ�����У�ʹ֮��Ӧ����I2��Ȼ����Na2S2O3����Һ�ζ�I2������������
����Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ���� ��
������I2�ķ�Ӧ�����ӷ���ʽ�� ��
��10�֣�ÿ��2�֣�
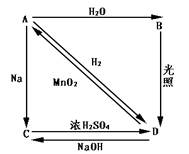
��1��

��2��Cl2 + 2OH�� Cl��+ ClO��+ H2O
Cl��+ ClO��+ H2O
��3��Ca(ClO)2 ="=" CaCl2 +O2��
��4���ٵ�����Һ �� ClO��+ 2I��+2H+��Cl��+ I2 + H2O
ClO��+ 2I��+2H+��Cl��+ I2 + H2O
��1��


��2��Cl2 + 2OH��
 Cl��+ ClO��+ H2O
Cl��+ ClO��+ H2O ��3��Ca(ClO)2 ="=" CaCl2 +O2��
��4���ٵ�����Һ ��
 ClO��+ 2I��+2H+��Cl��+ I2 + H2O
ClO��+ 2I��+2H+��Cl��+ I2 + H2O��

��ϰ��ϵ�д�
�����Ŀ
 ��
�� ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��
ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��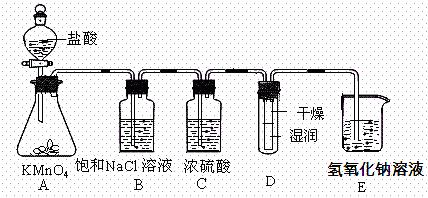
 __��
__�� ���µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________
���µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________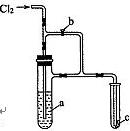
 ______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��
______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��  ����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________
����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________