��Ŀ����
��8�֣����ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ���������
��1���÷���ʽ��ʾ��ҵ����ʯ��ʯ������Ϊԭ������Ư�۵�ԭ��_________________
_________________________ ______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��
______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��
��2��Ư�ۿ���������ˮ��������������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬд����Ӧ�Ļ�ѧ����ʽ��___________________
��3��Ũ����ʹ��� ����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________
����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________
��1���÷���ʽ��ʾ��ҵ����ʯ��ʯ������Ϊԭ������Ư�۵�ԭ��_________________
_________________________
 ______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��
______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ�� ��2��Ư�ۿ���������ˮ��������������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬд����Ӧ�Ļ�ѧ����ʽ��___________________
��3��Ũ����ʹ���
 ����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________
����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________| A���٢ڢ� | B���ڢۢ� | C���ڢ� | D���٢� |

��

��ϰ��ϵ�д�
 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
�����Ŀ
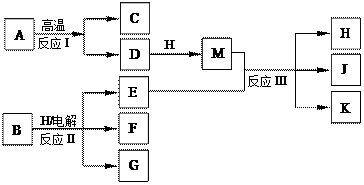
 E��G��Ӧ�����ӷ���ʽ�� ��
E��G��Ӧ�����ӷ���ʽ�� ��
 MnCl2��Cl2��+2H2O��
MnCl2��Cl2��+2H2O��