��Ŀ����
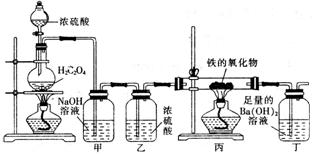
ij�����������ĩ���ܺ���FeO��Fe2O3�е�һ�ֻ����֣�һ��ѧ��ȤС��ͬѧ��������װ�òⶨ�������������ɣ���Э����ɣ����ش��й����⣮
��1����ͬѧ����1mol/L�����ᡢKSCN��Һ�����Ը��������Һ��ȷ������ɣ�
��2����ͬѧ�������Ϻ��Ϥ��H2C2O4
CO��+CO2��+H2O������������װ�ý��ж���̽����
��װ�ü�������
��ʵ�鿪ʼʱ����ͬѧ���ִ�Һ©��������Ũ���������£�ԭ����
����������������������Ϊ3.04g����ȫ��Ӧ�������ɳ���������Ϊ9.85g����ͨ������ȷ��������������ijɷּ������ʵ���֮�ȣ�
��1����ͬѧ����1mol/L�����ᡢKSCN��Һ�����Ը��������Һ��ȷ������ɣ�
| ��� | ʵ����� | ʵ����������� |
| �� | ȡ������ĩ�����Թ��У�ע��1mol/L������ | ��ĩ���ܽ⣬��Һ�ʻ���ɫ |
| �� | �����١���������Һ�ֳ����ݣ�������һ�ݵμӼ���KSCN��Һ���� | ����Һ��Ϊ Ѫ��ɫ Ѫ��ɫ ��˵����Fe2O3���� |
| �� | ����һ���м�������KMnO4��Һ | ����Һ ��ɫ��ȥ ��ɫ��ȥ ��˵����FeO���� |
| Ũ���� |
| �� |

��װ�ü�������
��ȥCO2
��ȥCO2
��װ�ö��е�ʵ�����������ְ�ɫ����
���ְ�ɫ����
����ʵ�鿪ʼʱ����ͬѧ���ִ�Һ©��������Ũ���������£�ԭ����
��Һ©���IJ�����û�д�
��Һ©���IJ�����û�д�
������������������������Ϊ3.04g����ȫ��Ӧ�������ɳ���������Ϊ9.85g����ͨ������ȷ��������������ijɷּ������ʵ���֮�ȣ�
��������1������Fe3+����KSCN��Һ����Һ��ΪѪ��ɫ��
�۸���KMnO4��Һ������Fe2+��������ɫ��ȥ��
��2���ٸ���H2C2O4�ֽ������������CO��CO2��H2O��װ�ü������dz�ȥCO2��װ���ҵ���������ˮ��װ�ñ�����CO��ԭ�����������������CO2��CO2����Ba��OH��2��Ӧ����̼�ᱵ������ˮ��
�ڸ��ݷ�Һ©���IJ�����û�д����Ũ���������£�
���ȸ��ݻ�ѧ����ʽ��CO2+Ba��OH��2=BaCO3��+H2O���ó��������������CO2���������ٸ��ݷ���ʽ��FexOy +yCO=xFe+yCO2������CO2�������������������������x��y��Ȼ����ݼ����жϳɷ֣�������FeO��Fe2O3����amol��bmol��
����
=
��������ʵ���֮�ȣ�
�۸���KMnO4��Һ������Fe2+��������ɫ��ȥ��
��2���ٸ���H2C2O4�ֽ������������CO��CO2��H2O��װ�ü������dz�ȥCO2��װ���ҵ���������ˮ��װ�ñ�����CO��ԭ�����������������CO2��CO2����Ba��OH��2��Ӧ����̼�ᱵ������ˮ��
�ڸ��ݷ�Һ©���IJ�����û�д����Ũ���������£�
���ȸ��ݻ�ѧ����ʽ��CO2+Ba��OH��2=BaCO3��+H2O���ó��������������CO2���������ٸ��ݷ���ʽ��FexOy +yCO=xFe+yCO2������CO2�������������������������x��y��Ȼ����ݼ����жϳɷ֣�������FeO��Fe2O3����amol��bmol��
����
| a+2b |
| a+3b |
| 4 |
| 5 |
����⣺��1����Fe3+����KSCN��Һ����Һ��ΪѪ��ɫ������Һ��ΪѪ��ɫ���١�����Һ����Fe3+��ԭ��������Fe2O3��
�ʴ�Ϊ����ɫ��
��KMnO4��Һ������Fe2+��������ɫ��ȥ��������һ���м�������KMnO4��Һ����ɫ��ȥ���١�����Һ����Fe2+��ԭ��������FeO���ʴ�Ϊ����ɫ��ȥ��
��2���ٸ���H2C2O4�ֽ������������CO��CO2��H2O��װ�ü������dz�ȥCO2��װ���ҵ���������ˮ��װ�ñ�����CO��ԭ�����������������CO2��CO2����Ba��OH��2��Ӧ����̼�ᱵ������ˮ��
�ʴ�Ϊ����ȥCO2�����ְ�ɫ������
�ڸ��ݷ�Һ©���IJ�����û�д����Ũ���������£�
�ʴ�Ϊ����Һ©���IJ�����û�д�
��COͨ����ԭ��������������CO2��װ�ö��е�ʵ�������DZ���ǣ�������ɫ������
CO2+Ba��OH��2=BaCO3��+H2O
44 197
X 9.85g
�������X����2.2������ԭ������CO2��2.2g��
FexOy +yCO=xFe+yCO2
56x+16y 44y
3.04 2.2g
���x��y=4��5��
����ij�����������ĩΪ��FeO��Fe2O3��
��FeO��Fe2O3����amol��bmol����
=
�����a��b=2��1��
�ʴ�Ϊ��FeO��Fe2O3��n��FeO����n��Fe2O3��=1��2��
�ʴ�Ϊ����ɫ��
��KMnO4��Һ������Fe2+��������ɫ��ȥ��������һ���м�������KMnO4��Һ����ɫ��ȥ���١�����Һ����Fe2+��ԭ��������FeO���ʴ�Ϊ����ɫ��ȥ��
��2���ٸ���H2C2O4�ֽ������������CO��CO2��H2O��װ�ü������dz�ȥCO2��װ���ҵ���������ˮ��װ�ñ�����CO��ԭ�����������������CO2��CO2����Ba��OH��2��Ӧ����̼�ᱵ������ˮ��
�ʴ�Ϊ����ȥCO2�����ְ�ɫ������
�ڸ��ݷ�Һ©���IJ�����û�д����Ũ���������£�
�ʴ�Ϊ����Һ©���IJ�����û�д�
��COͨ����ԭ��������������CO2��װ�ö��е�ʵ�������DZ���ǣ�������ɫ������
CO2+Ba��OH��2=BaCO3��+H2O
44 197
X 9.85g
�������X����2.2������ԭ������CO2��2.2g��
FexOy +yCO=xFe+yCO2
56x+16y 44y
3.04 2.2g
���x��y=4��5��
����ij�����������ĩΪ��FeO��Fe2O3��
��FeO��Fe2O3����amol��bmol����
| a+2b |
| a+3b |
| 4 |
| 5 |
�ʴ�Ϊ��FeO��Fe2O3��n��FeO����n��Fe2O3��=1��2��
������������Ҫ���������ʳɷֵļ���ͼ��㣬�ѶȽϴ���ʵ��Ŀ��ȷ����Ƶ�ʵ��װ�õ����ã��ǽ��ô�����Ļ�����ؼ���

��ϰ��ϵ�д�
�����Ŀ
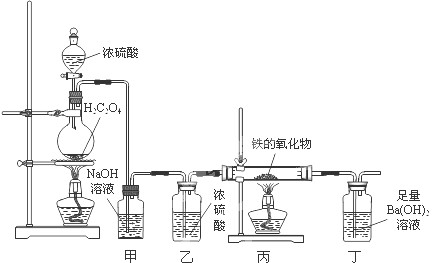
ij�����������ĩ���ܺ���FeO��Fe2O3�е�һ�ֻ����֣�һ��ѧ��ȤС��ͬѧ��������װ�òⶨ�������������ɣ���Э����ɣ����ش��й����⣮
��1����ͬѧ����1mol/L�����ᡢKSCN��Һ�����Ը��������Һ��ȷ������ɣ�
| ��� | ʵ����� | ʵ����������� |
| �� | ȡ������ĩ�����Թ��У�ע��1mol/L������ | ��ĩ���ܽ⣬��Һ�ʻ���ɫ |
| �� | �����١���������Һ�ֳ����ݣ�������һ�ݵμӼ���KSCN��Һ���� | ����Һ��Ϊ______��˵����Fe2O3���� |
| �� | ����һ���м�������KMnO4��Һ | ����Һ______��˵����FeO���� |
 CO��+CO2��+H2O������������װ�ý��ж���̽����
CO��+CO2��+H2O������������װ�ý��ж���̽����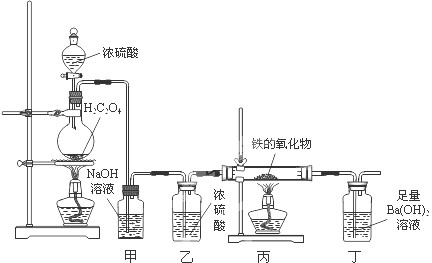
��װ�ü�������______��װ�ö��е�ʵ��������______��
��ʵ�鿪ʼʱ����ͬѧ���ִ�Һ©��������Ũ���������£�ԭ����______��
����������������������Ϊ3.04g����ȫ��Ӧ�������ɳ���������Ϊ9.85g����ͨ������ȷ��������������ijɷּ������ʵ���֮�ȣ�
 ��
�� ��
��
 ��������Ļ�ѧʽΪFexOy������з�Ӧ�Ļ�ѧ����ʽΪ ����������������������Ϊ3.92g������ȫ��Ӧ���������ɳ���������Ϊ13.79g����ȷ������������x : y= ���ù����ĩ�����Ϊ ��
��������Ļ�ѧʽΪFexOy������з�Ӧ�Ļ�ѧ����ʽΪ ����������������������Ϊ3.92g������ȫ��Ӧ���������ɳ���������Ϊ13.79g����ȷ������������x : y= ���ù����ĩ�����Ϊ ��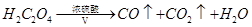 ��
��