��Ŀ����
(18��)ijУ��ѧʵ����ȤС���ڡ�̽��±�ص��ʵ������ԡ���ϵ��ʵ���з��֣���������ϡ�Ȼ�������Һ�У�����1��2����ˮ������Һ�ʻ�ɫ��
(1)������⣺ Fe3����Br2��һ���������Ը�ǿ��
(2)���룺
�ټ�ͬѧ��Ϊ�����ԣ�Fe3��>Br2��������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�________(�ѧʽ����ͬ)���¡�
����ͬѧ��Ϊ�����ԣ�Br2>Fe3����������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�__________���¡�
(3)���ʵ�鲢��֤��
��ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�ȷʵ����ȷ�ġ���ѡ�õ��Լ���
a����̪��Һ b��CCl4 c����ˮ�ƾ� d��KSCN��Һ
���������б�����д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������(�Լ������)
(4)Ӧ������չ
����������ϡ�Ȼ�������Һ�м���1��2����ˮ����Һ�ʻ�ɫ�������������ӷ�Ӧ����ʽΪ_____________________________________________________________��
����100 mL FeBr2��Һ��ͨ��2.24 L Cl2(��״��)����Һ����1/3��Br���������ɵ���Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ_________��
(1)������⣺ Fe3����Br2��һ���������Ը�ǿ��
(2)���룺
�ټ�ͬѧ��Ϊ�����ԣ�Fe3��>Br2��������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�________(�ѧʽ����ͬ)���¡�
����ͬѧ��Ϊ�����ԣ�Br2>Fe3����������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�__________���¡�
(3)���ʵ�鲢��֤��
��ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�ȷʵ����ȷ�ġ���ѡ�õ��Լ���
a����̪��Һ b��CCl4 c����ˮ�ƾ� d��KSCN��Һ
���������б�����д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������(�Լ������)
| | ѡ���Լ� | ʵ������ |
| ����1 | | |
| ����2 | | |
����������ϡ�Ȼ�������Һ�м���1��2����ˮ����Һ�ʻ�ɫ�������������ӷ�Ӧ����ʽΪ_____________________________________________________________��
����100 mL FeBr2��Һ��ͨ��2.24 L Cl2(��״��)����Һ����1/3��Br���������ɵ���Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ_________��
(17��,����ʽ3��,����ÿ��2��)
(2) ��Br2����Fe3����
(3) ����1��d����Һ�ʺ�ɫ�� ����2��b��CCl4�����ɫ��
(4) ��2Fe2����Br2===2Fe3����2Br���� ��1.2 mol/L
(2) ��Br2����Fe3����
(3) ����1��d����Һ�ʺ�ɫ�� ����2��b��CCl4�����ɫ��
(4) ��2Fe2����Br2===2Fe3����2Br���� ��1.2 mol/L
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 ɫ���____________ɫ��
ɫ���____________ɫ��
 ��֪��
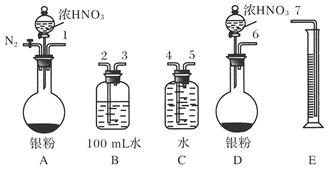
��֪�� ��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����
��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����


 ������ƽ��ҩ�ס����������___ _____�����������ƣ���
������ƽ��ҩ�ס����������___ _____�����������ƣ���
 MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���
MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���