��Ŀ����
 CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�꣮
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�꣮��1����һ�������£�CH4��CO2�����Ͻ�Ϊ������������Ӧ��CO2 ��g��+CH4��g��?2CO��g��+2H2��g������ƽ�ⳣ��ΪK���ڲ�ͬ�¶��£�K ��ֵ���£�
| �¶� | 200�� | 250�� | 300�� |
| K | 56 | 64 | 80 |
�ڴ��¶��¸÷�Ӧ��ƽ�ⳣ������ʽΪK=
��2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᣮ
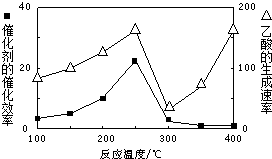
���ڲ�ͬ�¶��´����Ĵ�Ч���������������������ͼ��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ����
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
�۽�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ
��3��Li2O��Na2O��MgO��������CO2��
�����Ѱ������CO2���������ʣ����н����������
a�����ڼ�����������Ѱ��
b�����ڢ�A����A��Ԫ���γɵ���������Ѱ��
c�����ھ���ǿ�����Ե�������Ѱ��
��Li2O����CO2�������ںϳ�Li4SiO4��Li4SiO4�������ա��ͷ�CO2��ԭ���ǣ���500�棬CO2��Li4SiO4�Ӵ�������Li2CO3��ƽ��������700�棬��Ӧ������У��ų�CO2��Li4SiO4������˵����ԭ���Ļ�ѧ����ʽ��
���㣺��ѧƽ�ⳣ���ĺ���,��ѧ����ʽ����д,��ѧ��Ӧ���ʵ�Ӱ������
ר�⣺
��������1���ٷ���ͼ������ƽ�ⳣ�����¶�������������ӦΪ���ȷ�Ӧ���淴ӦΪ���ȷ�Ӧ��
��ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮����
��2���ٸ����¶ȶԴ������Ե�Ӱ�죻
�ڸ�����������Ի�ѧƽ���Ӱ�죬ƽ�������ƶ�����Ӧ��ת��������
���Ƚ�Cu2Al2O4������������ʽ��Cu2O?Al2O3���ٸ������������ᷴӦ�������ӷ���ʽ����Ҫע�����һ��ͭ���л�ԭ�ԣ�
��3���ٶ�����̼Ϊ�������壬Li2O��Na2O��MgO��������CO2���������أ�
�ڸ��������Ϣ����Ӧ��ΪCO2��Li4SiO4����������Li2CO3�����������غ���н��
��ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮����
��2���ٸ����¶ȶԴ������Ե�Ӱ�죻
�ڸ�����������Ի�ѧƽ���Ӱ�죬ƽ�������ƶ�����Ӧ��ת��������
���Ƚ�Cu2Al2O4������������ʽ��Cu2O?Al2O3���ٸ������������ᷴӦ�������ӷ���ʽ����Ҫע�����һ��ͭ���л�ԭ�ԣ�
��3���ٶ�����̼Ϊ�������壬Li2O��Na2O��MgO��������CO2���������أ�
�ڸ��������Ϣ����Ӧ��ΪCO2��Li4SiO4����������Li2CO3�����������غ���н��
���
�⣺��1���ٷ���ͼ������ƽ�ⳣ�����¶�������������ӦΪ���ȷ�Ӧ���淴ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ���ţ�
��CO2��g��+CH4��g��?2CO��g��+2H2��g������Ӧ��ƽ�ⳣ������ʽΪ��K=
��
�ʴ�Ϊ��
��
��2�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ������¶����߶�������������ʽ��ͣ�
�ʴ𰸣��¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ�
���������������Сѹǿ������CO2��Ũ�ȣ�ƽ�������ƶ�����Ӧ��ת��������
�ʴ�Ϊ�����������Сѹǿ������CO2��Ũ�ȣ�
��Cu2Al2O4������������ʽ��Cu2O?Al2O3�����ᷴӦ�������ӷ���ʽ��3Cu2Al2O4+32H++2NO3-=6Cu2++6Al3++2NO��+16H2O��
�ʴ�Ϊ��3Cu2Al2O4+32H++2NO3-=6Cu2++6Al3++2NO��+16H2O��
��3����a��Li2O��Na2O��MgO�����ڼ��������������������������CO2�����ڼ�����������Ѱ������CO2���������ʣ���a��ȷ��
b��Li2O��Na2O��MgO��������CO2���ơ�þ����Ϊ��A����A��Ԫ�أ����Կ��ڢ�A����A��Ԫ���γɵ���������Ѱ������CO2���������ʣ���b��ȷ��
c��Li2O��Na2O��MgO��������CO2�������Ƕ�û��ǿ�����ԣ������ն�����̼��������ԭ�أ���c����
�ʴ�Ϊ��ab��
����500�棬CO2��Li4SiO4�Ӵ�������Li2CO3����Ӧ��ΪCO2��Li4SiO4����������Li2CO3�����������غ��֪���ﻹ��Li2SiO3�����Ի�ѧ����ʽΪ��CO2+Li4SiO4
Li2CO3+Li2SiO3��
�ʴ�Ϊ��ab��CO2+Li4SiO4
Li2CO3+Li2SiO3��
�ʴ�Ϊ���ţ�
��CO2��g��+CH4��g��?2CO��g��+2H2��g������Ӧ��ƽ�ⳣ������ʽΪ��K=
| c2(CO)c2(H2) |
| c(CO2)c(CH4) |
�ʴ�Ϊ��
| c2(CO)c2(H2) |
| c(CO2)c(CH4) |
��2�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ������¶����߶�������������ʽ��ͣ�
�ʴ𰸣��¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ�
���������������Сѹǿ������CO2��Ũ�ȣ�ƽ�������ƶ�����Ӧ��ת��������
�ʴ�Ϊ�����������Сѹǿ������CO2��Ũ�ȣ�
��Cu2Al2O4������������ʽ��Cu2O?Al2O3�����ᷴӦ�������ӷ���ʽ��3Cu2Al2O4+32H++2NO3-=6Cu2++6Al3++2NO��+16H2O��
�ʴ�Ϊ��3Cu2Al2O4+32H++2NO3-=6Cu2++6Al3++2NO��+16H2O��
��3����a��Li2O��Na2O��MgO�����ڼ��������������������������CO2�����ڼ�����������Ѱ������CO2���������ʣ���a��ȷ��
b��Li2O��Na2O��MgO��������CO2���ơ�þ����Ϊ��A����A��Ԫ�أ����Կ��ڢ�A����A��Ԫ���γɵ���������Ѱ������CO2���������ʣ���b��ȷ��
c��Li2O��Na2O��MgO��������CO2�������Ƕ�û��ǿ�����ԣ������ն�����̼��������ԭ�أ���c����
�ʴ�Ϊ��ab��
����500�棬CO2��Li4SiO4�Ӵ�������Li2CO3����Ӧ��ΪCO2��Li4SiO4����������Li2CO3�����������غ��֪���ﻹ��Li2SiO3�����Ի�ѧ����ʽΪ��CO2+Li4SiO4
| 500�� |
| 700�� |
�ʴ�Ϊ��ab��CO2+Li4SiO4
| 500�� |
| 700�� |
������������Ҫ�������ۺ�����CO2���漰�Ȼ�ѧ��Ӧ���绯ѧ����ѧƽ��Ӱ�����صȣ���Ϊ�ۺϣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���ж���Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽ��Ԫ�صĵ縺�������ǣ�������
| A��1s22s22p2 |
| B��1s22s22p5 |
| C��1s22s22p63s23p4 |
| D��1s22s22p3 |
��ѧ���������ճ�������������Ҫ��Ӧ�ã�����������ȷ���ǣ�������
| A�����л������У������Ǻϳ����ϡ���������ά��ũҩ��Ⱦ�Ϻ�ҩƷ����Ҫԭ�� |
| B�����������������ڽ��ػ����������������ָ��£������������ǽ������� |
| C�����л��������ܻ������������ö�����������ͨ�����˷�����ȥ |
| D������β���к��е�������������Ͳ���ȫȼ����ɵ� |
����������ȷ���ǣ�������
| A��Li��������ȼ����Ҫ����Li2O2 |
| B����SO2ͨ��BaCl2��Һ������BaSO3���� |
| C����CO2ͨ����������Һ�����ɴ����� |
| D����COͨ���ȵ�CuSO4��Һ����ʹCu2+��ԭ��Cu |
�������������п��a��b�У��ֱ���������ϡH2SO4��ͬʱa�м�������CuSO4��Һ��ͼ�в��������������V����ʱ�䣨t���Ĺ�ϵ������ȷ���ǣ�������
A�� |
B�� |
C�� |
D�� |
����˵������ȷ���ǣ�������
| A��ij��Ӧ�ڵ������������Է����У���ô�÷�Ӧ�ڸ���������Ҳһ�����Է����� |
| B��ij��Ӧ�ڸ��������²����Է����У���ô�÷�Ӧ�ڵ���������Ҳһ�������Է����� |
| C����Ӧ���������ʱ���ر乲ͬ�����ģ��뷴Ӧ�¶��� |
| D���¶��п��ܶԷ�Ӧ�ķ�������������� |
������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
A�������µ��볣��ΪKa����HA ��Һ�� c ��H+��=
| ||
| B��0.2mol?L-1 CH3COOH��Һ��0.1mol?L-1NaOH��Һ��������2c��H+��-2c��OH-��=c��CH3COO-��-c��CH3COOH�� | ||
| C��������Na2SO4��Һ���뵽����ʯ��ˮ�У��а�ɫ����������˵��Ksp[Ca��OH��2]����Ksp��CaSO4�� | ||
| D�������£���0.1mol/L NH4HSO4��Һ�еμ�NaOH��Һ������c��Na+����c��NH4+����c��SO42-����c��OH-��=c��H+�� |
25�棬ˮ�ĵ���ﵽƽ�⣺H2O?H++OH-������������ȷ���ǣ�������
| A����ˮ�м�������ᣬƽ�������ƶ���c��H+������ |
| B����ˮ�м�����������̼�����ƣ�c��H+������Kw���� |
| C����ˮ�м�����������CH3COONa��ƽ�������ƶ���c��H+������ |
| D����ˮ���ȣ�Kw����pH���� |
���з�Ӧ����H++OH-�TH2O��ʾ���ǣ�������
| A��ϡ����������������Һ��Ӧ |
| B��ϡ����������������Һ��Ӧ |
| C������������������Һ��Ӧ |
| D������������������Ӧ |