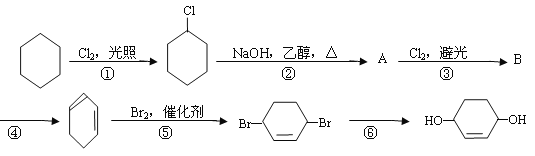
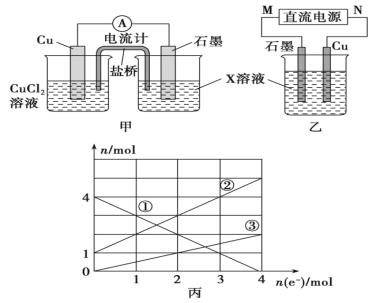
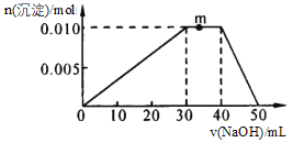
��Ŀ����
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ________��
��2��B���⻯��ķ��ӿռ乹����_____��������ԭ�Ӳ�ȡ___�ӻ���
��3��д��������AC2�ĵ���ʽ____��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ_____��
��4��ECl3��B��C���⻯���γ���λ��Ϊ���������Ļ�ѧʽΪ_____
���𰸡�C<O<N ������ sp3 ![]() N2O [Cr(NH3)4(H2O)2]Cl3
N2O [Cr(NH3)4(H2O)2]Cl3
��������
A��B��C��ͬһ���ڵķǽ���Ԫ�ء�AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе��������A��CԪ����B��NԪ�ء�C��OԪ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��D��MgԪ�أ�E��ԭ������Ϊ24��E��CrԪ����
(1) ͬһ����Ԫ�صĵ�һ�����ܴ������ҳ��������ƣ�����Nԭ��2p����������N�ĵ�һ�����ܴ���O�����Ե�һ��������С�����˳��ΪC<O<N���𰸣�C<O<N��
(2) ��������Nԭ�ӵļ۵��Ӷ�����![]() ����1�Թ¶Ե��ӣ������ӵĿռ乹���������Σ�������ԭ�Ӳ�ȡ���ӻ���ʽ��sp3���𰸣������Σ�sp3��
����1�Թ¶Ե��ӣ������ӵĿռ乹���������Σ�������ԭ�Ӳ�ȡ���ӻ���ʽ��sp3���𰸣������Σ�sp3��
(3)CO2�ĵ���ʽ![]() ���ȵ����������ԭ������ͬ���۵�����Ҳ��ͬ������һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O���𰸣�
���ȵ����������ԭ������ͬ���۵�����Ҳ��ͬ������һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O���𰸣�![]() ��N2O��
��N2O��
(4) ECl3���백����ˮ�γ���λ��Ϊ�������������������������ʵ���֮��Ϊ2:1������������λ�������ECl3�γɵ������Ļ�ѧʽΪ[Cr(NH3)4(H2O)2]Cl3���𰸣�[Cr(NH3)4(H2O)2]Cl3��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ��ij�о�С��Ϊ̽��SO2��Fe(NO3)3��Һ�ķ�Ӧ��ʵ�ʡ����������ͼ��ʾװ�ý���ʵ��.
��֪��1.0 mol/L��Fe(NO3)3��Һ��pH��1��

��ش�
��1��װ��A����������Ũ�������������Ϊ__________________��
��2��ʵ��ǰ����N2��Ŀ����________________________________��
��3��װ��B�в����˰�ɫ��������ɷ���________��˵��SO2����________�ԡ�
��4������B�в�����ɫ������ԭ��
�۵�1��SO2��Fe3����Ӧ��
�۵�2��������������SO2��NO3-��Ӧ��
�����۵�1��ȷ�������������⣬��Ӧ�۲쵽��������_________________��
�ڰ��۵�2��װ��B�з�Ӧ�����ӷ���ʽ��______________________________��
��������Ϊ���罫װ��B�е�Fe(NO3)3��Һ�滻Ϊ�������������Һ������ͬ�����½���ʵ�飬Ҳ����֤�۵�2�Ƿ���ȷ����ʱӦѡ�������Լ���(�����)_____��
A��1 mol/Lϡ���� |
B��1.5 mol/L Fe(NO3)2��Һ |
C��6.0 mol/L NaNO3��Һ��0.2 mol/L����������ϵ���Һ |
D��3.0 mol/L NaNO3��Һ��0.1mol/L����������ϵ���Һ |