��Ŀ����
����Ŀ�������Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ�������ܶ��ʻ���ˮ������ζ���������Ļ������ڿ���ͨ���˹������ϳɸ��������������ϡ��ǹ�����ˮ����ױƷ�е����ϣ�Ҳ��������ָ���͡���ˮ���ܼ�����ͼ�ǹ�ҵ����AΪ��Ҫԭ�����ϳ����������ĺϳ�·�ߡ�����A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����֪2CH3CHO+O2![]() 2CH3COOH����ش���������
2CH3COOH����ش���������
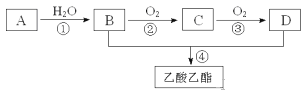
��1��B��D�����ں��еĹ����ŷֱ���_________��_________�������ƣ���
��2��д�����з�Ӧ�ķ�Ӧ���ͣ���______________����______________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��_______________________________________________________��
��____________________________________________________��
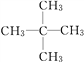
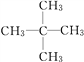
��3��A��H2�ڴ��������³�ַ�Ӧ����E��E��ͬϵ��F��E������̼ԭ�ӣ���д��F������ͬ���칹��Ľṹ��ʽ��________________________________________________��
���𰸡� �ǻ� �Ȼ� �ӳɷ�Ӧ ȡ����Ӧ��������Ӧ 2CH3CH2OH + O2 ![]() 2CH3CHO + 2H2O CH3COOH +CH3CH2OH
2CH3CHO + 2H2O CH3COOH +CH3CH2OH ![]() CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O ![]() ��
�� ![]() ��
�� 
�����������������A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪ��ϩ����ϩ��ˮ�ӳ������Ҵ����Ҵ�������������ȩ����ȩ�������������ᣬ���������Ҵ�����������Ӧ��������������B��C��D�ֱ�Ϊ�Ҵ�����ȩ�����ᡣ
��1��B��D�����ں��еĹ����ŷֱ����ǻ����Ȼ���
��2����Ӧ���ͣ��ټӳɷ�Ӧ����ȡ����Ӧ��������Ӧ��
��3����Ӧ�Ļ�ѧ����ʽ��
��2CH3CH2OH + O2 ![]() 2CH3CHO + 2H2O��
2CH3CHO + 2H2O��
��CH3COOH +CH3CH2OH ![]() CH3COOCH2CH3 + H2O��
CH3COOCH2CH3 + H2O��
��3��A��H2�ڴ��������³�ַ�Ӧ����E��EΪ���飬E��ͬϵ��F��E������̼ԭ�ӣ���F�Ƿ�������5��̼ԭ�ӵ������� F��ͬ���칹�干��3�֣���ṹ��ʽ�ֱ�Ϊ![]() ��
�� ![]() ��
��  ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�


