��Ŀ����
��A��B��C��D��E����Ԫ�أ�����A��B��C����ͬһ���ڣ���ԭ������B��C��Aԭ�������p�ܼ��ĵ��������ڴ����ĵ���������BԪ�ؿɷֱ���A��C��D��E���ɳ�����RB2�ͻ������֪��DB2��EB2�У�D��B��������Ϊ7��8��E��B��������Ϊ1��1�����������������ش��������⣺��1���ƶ�����Ԫ�طֱ��ǣ���Ԫ�����ƻش𣩣�D______��E______��
��2��ָ��CԪ����Ԫ�����ڱ��е�λ��______��EԪ�ص����������ķ��ӹ���Ϊ______��
��3���е�DB2______EB2�����������������=�����ڣ�������______
��4��д����EB3���ӻ�Ϊ�ȵ����岢��A��B��C��������Ԫ�ص���______����1�֣�
��5��ͨ�����ǰѲ�1molij��ѧ�������յ��������ɸû�ѧ���ļ��ܣ����ܵĴ�С���Ժ�����ѧ����ǿ����������һЩ���ļ��ܣ�SiCl4��g��+2H2��g�� ���� Si��s��+4HCl��g�� ͨ������÷�Ӧ��______�ȣ��������
���š���______kJ/mol
| ��ѧ�� | Si-O | Si-Cl | H-H | H-Cl | Si-Si | Si-C |
| ����/kJ?mol-1 | 460 | 360 | 436 | 431 | 176 | 347 |
���𰸡�������p�ܼ�������������6��Aԭ�������p�ܼ��ĵ��������ڴ����ĵ�������������A�ĺ�������Ų�Ϊ1s22s22p2��ӦΪCԪ�أ�BԪ�ؿɷֱ���A��C��D��E���ɳ�����RB2�ͻ����AΪCԪ�أ���CB2��BԪ�صĻ��ϼ�Ϊ-2�ۣ���A��B��C����ͬһ���ڣ���BΪOԪ�أ�CΪNԪ�أ����γ�NO2�������DB2��EB2�У�D��B��������Ϊ7��8������M��D����M��O��=7��4��M��D��=7×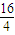 =28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ��ݴ˽����⣮
=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ��ݴ˽����⣮
����⣺p�ܼ�������������6��Aԭ�������p�ܼ��ĵ��������ڴ����ĵ�������������A�ĺ�������Ų�Ϊ1s22s22p2��ӦΪCԪ�أ�BԪ�ؿɷֱ���A��C��D��E���ɳ�����RB2�ͻ����AΪCԪ�أ���CB2��BԪ�صĻ��ϼ�Ϊ-2�ۣ���A��B��C����ͬһ���ڣ���BΪOԪ�أ�CΪNԪ�أ����γ�NO2�������DB2��EB2�У�D��B��������Ϊ7��8������M��D����M��O��=7��4��M��D��=7×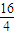 =28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ�
=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ�
��1��������������֪��DΪSiԪ�أ�EΪSԪ�أ��ʴ�Ϊ���裻��
��2��C��NԪ�أ���Ԫ�����ڱ��д��ڵڶ����ڵڢ�A�壬EԪ�ص����������ΪSO3��Sԭ�Ӽ۲���Ӷ�Ϊ3+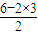 =3��û�й¶Ե��ӣ���Ϊƽ�������Σ�
=3��û�й¶Ե��ӣ���Ϊƽ�������Σ�
�ʴ�Ϊ���ڶ����ڵڢ�A�壻ƽ�������Σ�
��3��SiO2��ԭ�Ӿ��壬SO2�Ƿ��Ӿ��壬�ʷе�SiO2��SO2���ʴ�Ϊ������SiO2��ԭ�Ӿ��壬SO2�Ƿ��Ӿ��壬�е�SiO2��SO2��
��4����SO3���ӻ�Ϊ�ȵ����岢��C��O��N��������Ԫ�ص���ΪNO3-�ȣ��ʴ�Ϊ��NO3-��
��5������������������������״�ṹ��ÿ��Siԭ�ӳ�4��Si-Si����ÿ��Si-Si��Ϊ1��Siԭ���ṩ ����1mol�������Si-Si��Ϊ1mol×4×
����1mol�������Si-Si��Ϊ1mol×4× =2mol����ӦSiCl4��g��+2H2��g��=Si��s��+4HCl��g���ķ�Ӧ�ȡ�H=4×360kJ/mol+2×436kJ/mol-2×176kJ/mol-4×431kJ/mol=236kJ/mol����Ϊ���ȷ�Ӧ��
=2mol����ӦSiCl4��g��+2H2��g��=Si��s��+4HCl��g���ķ�Ӧ�ȡ�H=4×360kJ/mol+2×436kJ/mol-2×176kJ/mol-4×431kJ/mol=236kJ/mol����Ϊ���ȷ�Ӧ��
�ʴ�Ϊ������236��
���������⿼��Ԫ���ƶϡ�����ṹ�����ʡ���Ӧ�ȵ��йؼ���ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���ע�⣨5���з�Ӧ�ȵļ������״��㡢�ѵ㣬��ȷ�ж�1mol�������Si-Si���Ǽ���Ĺؼ���
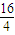 =28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ��ݴ˽����⣮
=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ��ݴ˽����⣮����⣺p�ܼ�������������6��Aԭ�������p�ܼ��ĵ��������ڴ����ĵ�������������A�ĺ�������Ų�Ϊ1s22s22p2��ӦΪCԪ�أ�BԪ�ؿɷֱ���A��C��D��E���ɳ�����RB2�ͻ����AΪCԪ�أ���CB2��BԪ�صĻ��ϼ�Ϊ-2�ۣ���A��B��C����ͬһ���ڣ���BΪOԪ�أ�CΪNԪ�أ����γ�NO2�������DB2��EB2�У�D��B��������Ϊ7��8������M��D����M��O��=7��4��M��D��=7×
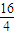 =28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ�
=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ���1��������������֪��DΪSiԪ�أ�EΪSԪ�أ��ʴ�Ϊ���裻��
��2��C��NԪ�أ���Ԫ�����ڱ��д��ڵڶ����ڵڢ�A�壬EԪ�ص����������ΪSO3��Sԭ�Ӽ۲���Ӷ�Ϊ3+
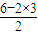 =3��û�й¶Ե��ӣ���Ϊƽ�������Σ�
=3��û�й¶Ե��ӣ���Ϊƽ�������Σ��ʴ�Ϊ���ڶ����ڵڢ�A�壻ƽ�������Σ�
��3��SiO2��ԭ�Ӿ��壬SO2�Ƿ��Ӿ��壬�ʷе�SiO2��SO2���ʴ�Ϊ������SiO2��ԭ�Ӿ��壬SO2�Ƿ��Ӿ��壬�е�SiO2��SO2��
��4����SO3���ӻ�Ϊ�ȵ����岢��C��O��N��������Ԫ�ص���ΪNO3-�ȣ��ʴ�Ϊ��NO3-��
��5������������������������״�ṹ��ÿ��Siԭ�ӳ�4��Si-Si����ÿ��Si-Si��Ϊ1��Siԭ���ṩ
 ����1mol�������Si-Si��Ϊ1mol×4×
����1mol�������Si-Si��Ϊ1mol×4× =2mol����ӦSiCl4��g��+2H2��g��=Si��s��+4HCl��g���ķ�Ӧ�ȡ�H=4×360kJ/mol+2×436kJ/mol-2×176kJ/mol-4×431kJ/mol=236kJ/mol����Ϊ���ȷ�Ӧ��
=2mol����ӦSiCl4��g��+2H2��g��=Si��s��+4HCl��g���ķ�Ӧ�ȡ�H=4×360kJ/mol+2×436kJ/mol-2×176kJ/mol-4×431kJ/mol=236kJ/mol����Ϊ���ȷ�Ӧ���ʴ�Ϊ������236��
���������⿼��Ԫ���ƶϡ�����ṹ�����ʡ���Ӧ�ȵ��йؼ���ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���ע�⣨5���з�Ӧ�ȵļ������״��㡢�ѵ㣬��ȷ�ж�1mol�������Si-Si���Ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ





