��Ŀ����
һ���¶��£��п��淴Ӧ��2X(g)��2Y(g)![]() Z(g)��3P(g)����H��0���ֽ�2molX��2molY�������ΪV�ļ���������2molZ��6molP������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V(��ͼ��ʾ)�����ַ�Ӧ���������������¶�����ʼʱ��ͬ������˵����ȷ���ǣ� ��
Z(g)��3P(g)����H��0���ֽ�2molX��2molY�������ΪV�ļ���������2molZ��6molP������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V(��ͼ��ʾ)�����ַ�Ӧ���������������¶�����ʼʱ��ͬ������˵����ȷ���ǣ� ��
 A������������ٳ���2molX��2molY��ƽ����������Z��Ũ�Ȳ���
A������������ٳ���2molX��2molY��ƽ����������Z��Ũ�Ȳ���
B���ס����������еķ�Ӧ�ﵽ��ѧƽ��ʱ��Z������������
C���ס����������еķ�Ӧ�ﵽ��ѧƽ��ʱ����������ѹǿ��
D�������������ٳ���1molZ��3molP��ƽ�����������Z��Ũ��Ϊԭ����1.5��
B
����:
ע����ݻ�������ҵ��ݻ��ɱ䣬��Ӧǰ����������ʵ����Dz���ķ�Ӧ

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
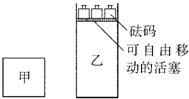 һ���¶��£��п��淴Ӧ��2A��g��+2B��g��?C��g��+3D��g������H��0���ֽ�2molA��2molB�������ΪV�ļ���������2molC��6molD������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ��ʾ�������ַ�Ӧ���������������¶�����ʼʱ��ͬ������˵����ȷ���ǣ�������
һ���¶��£��п��淴Ӧ��2A��g��+2B��g��?C��g��+3D��g������H��0���ֽ�2molA��2molB�������ΪV�ļ���������2molC��6molD������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ��ʾ�������ַ�Ӧ���������������¶�����ʼʱ��ͬ������˵����ȷ���ǣ�������| A���ס����������еķ�Ӧ�ﵽ��ѧƽ��ʱ����������ѹǿ��� | B���ס����������еķ�Ӧ�ﵽ��ѧƽ��ʱ��C������������ | C������������ٳ���2molA��2molB��ƽ����������C��Ũ�Ȳ��� | D�������������ٳ���2molC��6molD��ƽ�����������C��Ũ��Ϊԭ����2�� |



 ��2009?����һģ��һ���¶��£��п��淴Ӧ��3Fe��s��+4H2O��g��?Fe3O4��s��+4H2 ��g����H��0������˵������ȷ���ǣ�������
��2009?����һģ��һ���¶��£��п��淴Ӧ��3Fe��s��+4H2O��g��?Fe3O4��s��+4H2 ��g����H��0������˵������ȷ���ǣ�������