��Ŀ����
16��A��B��C��D��E���������������е�����Ԫ�أ����ǵ�ԭ��������������B��C��D��ͬһ���ڣ�A��E�����ڱ��д���ͬһ���У���֪���ٳ�A�⣬��Ԫ��ԭ�ӵ��ڲ�����������ӣ�����B���������4�����ӣ�
��A��B��B��C����������̬�Ǽ��Է��ӵĻ����
��D��E�������ӻ���������ӵĵ��Ӳ�ṹ��ͬ���ش�
��1��AΪHԪ�أ�BΪCԪ�أ�CΪOԪ�أ�DΪFԪ�أ�
��2��CԪ�������ڱ����������ڢ�A��Ԫ�أ�ԭ�ӽṹ��ͼΪ
 ��
���ִ�ԭ�ӽṹ������Ϊ����ͬһ���Ӳ��ϣ�����s��p��d��f��g��h�����ܼ������ܼ��ֱ���1��3��5������������Ը��ݵ�����������˳��Ԥ�⣺
��3����8���ڹ���50��Ԫ�أ�
��4��ԭ�Ӻ�����ֵ�һ��6f���ӵ�Ԫ�ص�ԭ��������139��
��5�����ݡ��ȶ�������˵����114��Ԫ����һ���ȶ�ͬλ�أ���˥�ںܳ�����������Ȼ�綼�����ҵ������Ʋ��114��Ԫ�����������ڣ���A��Ԫ�أ�ԭ�ӵ���Χ�����Ų�ʽ��7s27p2��
���� ��1��A��B��C��D��E���������������е�����Ԫ�أ��ٳ�A�⣬��Ԫ��ԭ�ӵ��ڲ�����������ӣ���AΪHԪ�أ�B��C��D��ͬһ���ڣ������ڵڶ����ڣ�B���������4�����ӣ���BΪ̼Ԫ�أ�A��E�����ڱ��д���ͬһ���У�����֪EΪNaԪ�أ���A��B��B��C����������̬�Ǽ��Է��ӵĻ������֪CΪOԪ�أ��γɵķǼ��Է���ΪCH4��CO2��D��E�������ӻ���������ӵĵ��Ӳ�ṹ��ͬ����DΪFԪ�أ�
��2��CΪOԪ�أ�ԭ�Ӻ�����2�����Ӳ㣬���������Ϊ2��6������Ԫ��������=���Ӳ���������������=������������
��3�����ڣ�n-3��g����n-2��f����n-1��d��n s��n p���ʵڰ����ں���s��p��d��f��g�ܼ������ܼ����ɵ�����Ŀ֮��Ϊ�ø���������Ԫ��������
��4��ԭ�Ӻ�����ֵ�һ��6f���ӵ�Ԫ�ص�ԭ����Χ�����Ų���5g186f18s2�����ݺ�������Ų�ʽ��Ӧ���ǣ�[118]5g186f18s2��
��5��������������Ԫ�ص�ԭ��������118������114��Ԫ���ڴ�Ԫ�ص���ߣ�Ӧ���ǵ������ڢ�A��Ԫ�أ�
��� �⣺��1��A��B��C��D��E���������������е�����Ԫ�أ��ٳ�A�⣬��Ԫ��ԭ�ӵ��ڲ�����������ӣ���AΪHԪ�أ�B��C��D��ͬһ���ڣ������ڵڶ����ڣ�B���������4�����ӣ���BΪ̼Ԫ�أ�A��E�����ڱ��д���ͬһ���У�����֪EΪNaԪ�أ���A��B��B��C����������̬�Ǽ��Է��ӵĻ������֪CΪOԪ�أ��γɵķǼ��Է���ΪCH4��CO2��D��E�������ӻ���������ӵĵ��Ӳ�ṹ��ͬ����DΪFԪ�أ�
�ʴ�Ϊ��H��C��O��F��
��2��CΪOԪ�أ�ԭ�Ӻ�����2�����Ӳ㣬���������Ϊ2��6��ԭ�ӽṹʾ��ͼΪ �����ڵڶ��ܢ�A�壬
�����ڵڶ��ܢ�A�壬
�ʴ�Ϊ��������A�� ��
��
��3�����ڣ�n-3��g����n-2��f����n-1��d��n s��n p���ʵڰ����ں���s��p��d��f��g�ܼ������ܼ����ɵ�����Ŀ֮��Ϊ�ø���������Ԫ��������������Ԫ������Ϊ��1��2+3��2+5��2+7��2+9��2=50��
�ʴ�Ϊ��50��
��4��ԭ�Ӻ�����ֵ�һ��6f���ӵ�Ԫ�ص�ԭ����Χ�����Ų���5g186f18s2�����ݺ�������Ų�ʽ��Ӧ���ǣ�[118]5g186f18s2������139��Ԫ�أ��ʴ�Ϊ��139��
��5��������������Ԫ�ص�ԭ��������118������114��Ԫ���ڴ�Ԫ�ص���ߣ�Ӧ���ǵ������ڣ���A��Ԫ�أ�����p��Ԫ�أ��۵����Ų��ǣ�7s27p2��
�ʴ�Ϊ���ߣ���A��7s27p2��
���� �����ǶԽṹ��ѧ֪ʶ�Ŀ��飬����Ԫ��ԭ�ӹ���ܼ���֪ʶ���飬�ϺõĿ���ѧ�����������������ѶȽϴ�

| A�� | HR | B�� | H2R | C�� | RH3 | D�� | RH4 |
| A�� | ��״���£�22.4L H2���е�ԭ����ΪNA | |
| B�� | ��������O2��O3����������ԭ������� | |
| C�� | ͬ��ͬѹ�£���ͬ�����H2��O2����������Ϊ1��16 | |
| D�� | 28g CO��22.4L CO2������̼ԭ������� |
| ��ѧ�� | A-A | B-B | A-B |
| ����1mol��ѧ��ʱ�ų������� | 436kJ•mol-1 | 243kJ•mol-1 | 431kJ•mol-1 |
| A�� | $\frac{1}{2}$A2��g��+$\frac{1}{2}$B2��g���TAB��g����H=-91.5 kJ•mol -1 | |
| B�� | A2��g��+B2��g���T2AB��g����H=-183 kJ•mol -1 | |
| C�� | $\frac{1}{2}$A2��g��+$\frac{1}{2}$B2�TAB��g����H=+91.5 kJ•mol-1 | |
| D�� | 2AB��g���TA2��g��+B2��g����H=+183 kJ•mol-1 |
��1����ˮ����ͨ�����ȵ�̼���ɲ���ˮú����
��ӦΪ��C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1��
�ٸ÷�Ӧ�ڸ��������Է����У���ܡ����ܡ�����
�ں����£����ݻ�������ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬���У�D��
����������ܶȣ��������������ѹǿ����������������ʵ�������CO���ʵ���Ũ��
A��ֻ�Т�B��ֻ�Т�͢�C��ֻ�Т�͢�D����͢�
��2��ˮú���ٽ�һ����Ӧ����ȡ��������ӦΪH2O��g��+CO��g��?H2��g��+CO2��g����ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2��g����CO2��g��������ʼŨ�����±���ʾ�������жϲ���ȷ����C��
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mol/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/mol/L | 0.010 | 0.010 | 0.020 |
B��ƽ��ʱ�����кͱ���H2��ת���ʾ���60%
C��ƽ��ʱ������c��CO2���Ǽ��е�2������0.012mol/L
D��ƽ��ʱ������CO2��ת���ʴ���60%
 ijͬѧ������װ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣮
ijͬѧ������װ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣮
 ��
�� ��
�� ��
�� ������NaOH��Һ��Ӧʱ���������4mol NaOH
������NaOH��Һ��Ӧʱ���������4mol NaOH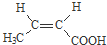 ��
�� ��
��